Cleavage (Fission Or Breaking) Of Covalent Bonds
Category : JEE Main & Advanced
Breaking of covalent bond of the compound is known as bond fission. A bond can be broken by two ways,
(1) Homolytic bond fission or Homolysis
(i) In homolysis, the covalent bond is broken in such a way that each resulting species gets its own electron. This leads to the formation of odd electron species known as free radical.
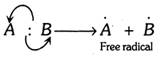
(ii) The factor which favours homolysis is that the difference in electronegativity between \[A\] and \[B\] is less or zero.
(iii) Homolysis takes place in gaseous phase or in the presence of non polar solvents \[(CC{{l}_{4}},\,C{{S}_{2}})\], peroxide, \[UV\] light, heat \[(\ge {{500}^{o}}C)\], electricity and free radical.
(iv) Mechanism of the reaction in which homolysis takes place is known as homolytic mechanism or free radical mechanism.
(2) Heterolytic bond fission or heterolysis
(i) In heterolysis, the covalent bond is broken in such a way that one species (i.e., less electronegative) is deprived of its own electron, while the other species gains both the electrons.
![]()
Thus formation of opposite charged species takes place. In case of organic compounds, if positive charge is present on the carbon then cation is termed as carbocation. If negative charge is present on the carbon then anion is termed as carbanion.
(ii) The factor which favours heterolysis is greater difference of electronegativities between \[A\] and \[B\].
(iii) Mechanism of the reaction in which heterolysis takes place is known as heterolytic mechanism or ionic mechanism.
(iv) The energy required for heterolysis is always greater than that for homolysis due to electrostatic forces of attraction between ions.
You need to login to perform this action.
You will be redirected in
3 sec
