Types Of Organic Reactions
Category : JEE Main & Advanced
It is convenient to classify the numerous reactions of the various classes of organic compound into four types,
Substitution reactions
Replacement of an atom or group of the substrate by any other atom or group is known as substitution reactions.
Examples :
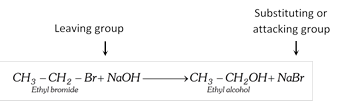
(Bromine atom is replaced by hydroxyl group)
Types of substitution reactions : On the basis of the nature of attacking species substitution reactions are classified into following three categories,
(1) Nucleophilic substitution reactions
(2) Electrophilic substitution reactions
(3) Free radical substitution reactions
(1) Nucleophilic substitution reactions
(i) Many substitution reactions, especially at the saturated carbon atom in aliphatic compounds such as alkyl halides, are brought about by nucleophilic reagents or nucleophiles.
\[\underset{Substrate}{\mathop{R-X}}\,+\underset{Nucleophile}{\mathop{O{{H}^{\Theta }}}}\,\xrightarrow{\,\,\,\,\,\,\,\,\,}R-OH+\underset{Leaving\,group}{\mathop{{{X}^{\Theta }}}}\,\]
Such substitution reactions are called nucleophilic substitution reactions, i.e., \[{{S}_{N}}\]reactions (S stands for substitution and N for nucleophile).
(ii) The weaker the basicity of a group of the substrate, the better is its leaving ability.
Leaving power of the group \[\propto \frac{1}{\text{Basicity}\,\text{of}\,\text{the}\,\text{group}}\]
Example :
\[\underset{\text{Decreasing acidity}}{\mathop{\xrightarrow{HI>HBr>HCl>HF}}}\,\]
\[\underset{\underset{\underset{\text{Decreasing}\,\text{leaving}\,\text{ability}}{\mathop{\Downarrow }}\,}{\mathop{\text{Increasing basicity}}}\,}{\mathop{\xrightarrow{\,\,\,\,\,\overset{\Theta }{\mathop{I}}\,\,\overset{\Theta }{\mathop{\,\,\,\,\,Br\,\,\,\,\,\,}}\,\overset{\Theta }{\mathop{Cl}}\,\,\,\,\,\,\,\overset{\Theta }{\mathop{F}}\,\,\,\,\,\,\,\,}}}\,\]
(iii) The leaving power of some nucleophilic groups are given below in decreasing order,
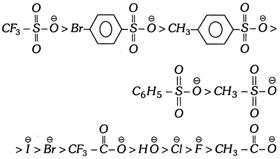
(iv) In these reactions leaving group of the substrate is replaced by another nucleophile. If reagent is neutral then leaving group is replaced by negative part of the reagent. Negative part of the reagent is always nucleophilic in character.
\[R-L\xrightarrow{\overset{+\delta }{\mathop{E}}\,-\overset{-\delta }{\mathop{Nu}}\,}R-Nu+\overset{\Theta }{\mathop{L}}\,\]; \[R-L+\overset{\Theta }{\mathop{Nu}}\,\xrightarrow{{}}R-Nu+\overset{\Theta }{\mathop{L}}\,\]
(v) In \[{{S}_{N}}\] reactions basicity of leaving group should be less than the basicity of incoming nucleophilic group. Thus strongly basic nucleophilic group replaces weakly basic nucleophilic group of the substrate.
Example :
\[R-Cl\underset{(NaOH)}{\mathop{\xrightarrow{O{{H}^{\Theta }}}}}\,R-OH+\overset{\Theta }{\mathop{Cl}}\,\] .....(A)
Basicity of \[O{{H}^{\Theta }}\]is more than\[\overset{\Theta }{\mathop{Cl}}\,\]hence\[\overset{\Theta }{\mathop{OH}}\,\]replaces Cl as \[\overset{\Theta }{\mathop{Cl}}\,\].
\[R-OH\underset{(HCl)}{\mathop{\xrightarrow{\overset{\Theta }{\mathop{Cl}}\,\,}}}\,R-Cl+\overset{\Theta }{\mathop{OH}}\,\] ......(B)
Basicity of \[\overset{\Theta }{\mathop{Cl}}\,\] is less than \[\overset{\Theta }{\mathop{OH}}\,\], hence \[\overset{\Theta }{\mathop{Cl}}\,\] will not replace OH as \[\overset{\Theta }{\mathop{OH}}\,\] hence reaction (B) will not occur.
(vi) Unlike aliphatic compounds having nucleophilic group as leaving group, aromatic compounds having same group bonded directly with aromatic ring do not undergo nucleophilic substitution reaction under ordinary conditions.
The reason for this unusual reactivity is the presence of lone pair of electron or \[\pi \] bond on the key atom of the functional group. Another factor for the low reactivity is nucleophilic character of aromatic ring.
(vii) The \[{{S}_{N}}\] reactions are divided into two classes, \[{{S}_{{{N}^{2}}}}\] and \[{{S}_{{{N}^{1}}}}\] reactions.
Distinction between \[{{S}_{{{N}^{2}}}}\] and \[{{S}_{{{N}^{1}}}}\] reactions
| Factors | \[{{S}_{{{N}^{2}}}}\] Reactions | \[{{S}_{{{N}^{1}}}}\] Reactions |
| Number of steps | One: \[R:L+:\overset{\Theta }{\mathop{Nu}}\,\to R:Nu+:\overset{\Theta }{\mathop{L}}\,\] |
Two: (i) \[R:L\xrightarrow{Slow}{{R}^{+}}+:{{L}^{}}\] (ii) \[{{R}^{+}}+:N{{u}^{-}}\xrightarrow{Fast}R:Nu\] |
| Reaction rate and order | Second order: Rate \[\propto \] [Substrate] [Nucleophile] or Rate =\[{{K}_{2}}[RL][:N{{u}^{}}]\] | First order: Rate \[\propto \] [Substrate] or Rate =\[{{K}_{1}}[RL]\] |
| Molecularity | Bimolecular | Unimolecular |
| TS of slow step | \[:\overset{{{\delta }^{-}}}{\mathop{Nu}}\,---C---:\overset{{{\delta }^{-}}}{\mathop{L}}\,\] | \[:Nu---\overset{{{\delta }^{+}}}{\mathop{C}}\,---\overset{{{\delta }^{-}}}{\mathop{L}}\,---Nu:\] |
| Reacting nucleophile | The nucleophile attacks the carbon of the substrate exclusively from the back side. | The nucleophile can attack the carbon of the substrate both from the back and front sides although the back side attack predominates. |
| Stereochemistry | Complete inversion of configuration takes place. | Inversion and retention takes place. |
| Reactivity order of alkyl halides | Methyl>1°>2°>3°halides. \[(I>Br>Cl>F)\] | 3°>2°>1° > methyl halides.\[(I>Br>Cl>F)\] |
| Rearrangement | No rearranged product is formed (except for allylic). | Rearranged products can be formed. |
| Nature of nucleophiles | Favoured by strong and high concentration of nucleophiles. | Favoured by mild and low concentration of nucleophiles. |
| Polarity | Favoured by solvents of low polarity. | Favoured by solvents of high polarity. |
| Reaction rate determining factor | By steric hindrance. | By electronic factor (stability of \[\overset{\oplus }{\mathop{R}}\,\]). |
| Catalysis | Not catalysed by any catalyst (phase transfer). | Catalysed by Lewis and Bronsted acids, e.g., \[\overset{\oplus }{\mathop{Ag}}\,,AlC{{l}_{3}},ZnC{{l}_{2}}\]and strong HA. |
(2) Electrophilic substitutions reactions : Electrophilic substitution involves the attack by an electrophile. It is represented as SE (S stands for substitution and E stands for elctrophile). If the order of reaction is 1, it is written as \[{{S}_{{{E}^{1}}}}\] (unimolecular)and if the order is 2, it is \[{{S}_{{{E}^{2}}}}\] (Bimolecular).
|
SE1 Reaction mechanism : Electrophilic substitution in aliphatic compounds are very rare; some of the important examples are: (i) Replacement of the metal atom in an organometallic compound by hydrogen : \[R-M+\overset{\oplus }{\mathop{H}}\,\to R-H+\overset{\oplus }{\mathop{M}}\,\] e.g., \[C{{H}_{3}}-C{{H}_{2}}-MgBr\xrightarrow{-MgBr}C{{H}_{3}}-{{\overset{\Theta }{\mathop{CH}}\,}_{2}}\xrightarrow{\overset{\oplus }{\mathop{H}}\,}C{{H}_{3}}-C{{H}_{3}}\] \[C{{H}_{3}}-C{{H}_{2}}-MgBr+H-Br\to C{{H}_{3}}-{{\overset{\Theta }{\mathop{CH}}\,}_{2}}\xrightarrow{\overset{\oplus }{\mathop{H}}\,}C{{H}_{3}}-C{{H}_{3}}+MgB{{r}_{2}}\] \[C{{H}_{3}}-C{{H}_{2}}Na+{{C}_{6}}{{H}_{6}}\to C{{H}_{3}}-C{{H}_{3}}+{{C}_{6}}{{H}_{5}}Na\] (ii) Decarboxylation of silver salt of carboxylic acid by means of bromine: \[{{R}_{3}}C-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-OAg+Br-Br\to {{R}_{3}}C-\underset{O}{\mathop{\underset{||}{\mathop{C}}\,}}\,-\overset{\Theta }{\mathop{O}}\,+\overset{{{\delta }^{+}}}{\mathop{Br}}\,-\overset{{{\delta }^{-}}}{\mathop{Br}}\,+\overset{\oplus }{\mathop{Ag}}\,\] \[\to {{R}_{3}}C-Br+C{{O}_{2}}+AgBr\] (iii) Isotopic exchange of hydrogen for deuterium or tritium: \[R-H+\overset{\oplus }{\mathop{D}}\,\rightleftharpoons R-D+\overset{\oplus }{\mathop{H}}\,\] \[R-H+\overset{\oplus }{\mathop{T}}\,\rightleftharpoons R-T+\overset{\oplus }{\mathop{\text{H}}}\,\]
|
SE2 Reaction mechanism : Electrophilic substitution is very common in benzene nucleus (aromatic compounds) in which p-electrons are highly delocalized and an electrophile can attack this region of high electron density. In all electrophilic aromatic substitution reactions, it involves: Step 1. The formation of an electrophile, \[\overset{\oplus }{\mathop{E}}\,,\]i.e., In halogenation; \[Cl-Cl+FeC{{l}_{3}}\to \overset{\oplus }{\mathop{Cl}}\,+Fe{{\overset{\Theta }{\mathop{Cl}}\,}_{4}}\] In nitration; \[HN{{O}_{3}}+2{{H}_{2}}S{{O}_{4}}\to {{\overset{\oplus }{\mathop{NO}}\,}_{2}}+2{{\overset{\Theta }{\mathop{HSO}}\,}_{4}}+\overset{\oplus }{\mathop{{{H}_{3}}O}}\,\] In sulphonation; \[2{{H}_{2}}S{{O}_{4}}\to S{{O}_{3}}+{{\overset{\Theta }{\mathop{HSO}}\,}_{4}}+\overset{\oplus }{\mathop{{{H}_{3}}O}}\,\] In Friedel-crafts reaction; \[R-Cl+AlC{{l}_{3}}\to \overset{\oplus }{\mathop{R}}\,+{{\overset{\Theta }{\mathop{AlCl}}\,}_{4}}\] \[RCOCl+AlC{{l}_{3}}\to \overset{\oplus }{\mathop{RCO}}\,+{{\overset{\Theta }{\mathop{AlCl}}\,}_{4}}\] Step 2. The electrophile attacks the aromatic ring to form carbonium ion (or arenium ion) which is stabilized by resonance.
Step 3. Carbonium ion loses the proton to form substitution product.
The bromination of benzene in the presence of \[FeB{{r}_{3}}\]is a example of electrophilic substitution reaction. Similarly, Nitration, sulphonation and Friedel-Crafts reaction…..etc., in benzene nucleus are the other examples of electrophilic substitution reactions. |
(3) Free radical substitution reactions : Free radical substitution reactions involves the attack by a free radical. These reactions occurs by free radical mechanism which involves Initiation, Propagation and Termination steps. Examples,
(i) Chlorination of methane : The chlorination of methane in the presence of ultraviolet light is an examples of free radical substitution.
\[\underset{Methane}{\mathop{C{{H}_{4}}}}\,+C{{l}_{2}}\underset{light}{\mathop{\xrightarrow{UV}}}\,\underset{\text{Methyl}\,\text{chloride}}{\mathop{C{{H}_{3}}Cl}}\,+HCl\]
(ii) Arylation of aromatic compounds (Gomberg reaction) : The reaction of benzene diazonium halide with benzene gives diphenyl by a free radical substitution reaction.
\[{{C}_{6}}{{H}_{5}}-H+\underset{\text{Benzene}\,\text{diazonium}\,\text{halide}}{\mathop{{{C}_{6}}{{H}_{5}}{{N}_{2}}X}}\,\xrightarrow{\text{Alkali}}\underset{\text{Diphenyl}}{\mathop{{{C}_{6}}{{H}_{5}}-{{C}_{6}}{{H}_{5}}}}\,+{{N}_{2}}+HX\]
(iii) Wurtz reaction : Ethyl bromide on treatment with metallic sodium forms butane, ethane and ethylene by involving free radical mechanism.
(iv) Allylic bromination by NBS (N-Bromosuccinimide) : NBS is a selective brominating agent and it normally brominates the ethylenic compounds in the allylic \[(C{{H}_{2}}=CH-C{{H}_{2}}-)\] position. This type of reaction involving substitution at the alpha carbon atom with respect to the double bond is termed Allylic substitution. It is also used for benzylic bromination. Some examples are:

You need to login to perform this action.
You will be redirected in
3 sec
