General characteristics of Halo-Alkenes
Category : JEE Main & Advanced
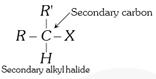
General characteristics of Halo-Alkenes (1) Organic compounds in which halogen atom \[(F,Cl,Br,I)\] is directly linked with saturated carbon atom are known as halo-alkanes. General formula is \[{{C}_{n}}{{H}_{2n+2-m}}{{X}_{m}}\] (\[X=F,Cl,Br,I\]) and \[m=\text{no}\text{.}\]of halogen atom; \[n=\text{no}\text{.}\]of carbon atoms. (2) Depending on the number of halogen atoms present in the halogen derivative, these are termed as mono-, di-, tri-, tetra-, and polyhalogen derivatives. \[\left[ \underset{\text{Methane}}{\mathop{C{{H}_{4}}}}\,\underset{+X}{\mathop{\xrightarrow{-H}}}\,\underset{\text{Mono}}{\mathop{C{{H}_{3}}-X}}\,\underset{+X}{\mathop{\xrightarrow{-H}}}\,\underset{\text{Di}}{\mathop{C{{H}_{2}}-{{X}_{2}}}}\,\underset{+X}{\mathop{\xrightarrow{-H}}}\,\underset{\text{Tri}}{\mathop{CH-{{X}_{3}}}}\,\underset{+X}{\mathop{\xrightarrow{-H}}}\,\underset{\text{Tetra}}{\mathop{C-{{X}_{4}}}}\, \right]\] (i) Monohalogen derivatives are termed as alkyl halides. Example: \[\underset{\text{Methyl}\,\text{chloride}}{\mathop{C{{H}_{3}}Cl}}\,\] \[\underset{\text{Ethyl}\,\text{bromide}}{\mathop{{{C}_{2}}{{H}_{5}}Br}}\,\] \[\underset{\text{Propyl}\,\text{iodide}}{\mathop{{{C}_{3}}{{H}_{7}}I}}\,\] Monohalogen derivatives or alkyl halides are classified as primary (1°), secondary (2°) or tertiary (3°) depending upon whether the halogen atom is attached to primary, secondary or tertiary carbon atoms. 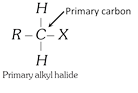
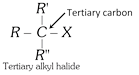 (ii) The dihalogen derivatives are mainly of three types (a) Gem-dihalides: In these derivatives both the halogen atoms are attached to the same carbon atom. These are also called alkylidene halides.
(ii) The dihalogen derivatives are mainly of three types (a) Gem-dihalides: In these derivatives both the halogen atoms are attached to the same carbon atom. These are also called alkylidene halides. 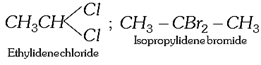 (b) Vic-dihalides : In these derivatives, the halogen atoms are attached to adjacent (Vicinal) carbon atoms. These are also termed as alkylene halides. \[\underset{\text{Ethylene}\,\text{chloride}}{\mathop{C{{H}_{2}}Cl.C{{H}_{2}}Cl}}\,\] ; \[\underset{\text{Propylene}\,\text{chloride}}{\mathop{C{{H}_{3}}CHCl.C{{H}_{2}}Cl}}\,\] (c) a-w halides (Terminal dihalides): In these derivatives, the halogen atoms are attached to terminal carbon atoms. These are also called polymethylene halides. \[\underset{\text{Trimethylene bromide }}{\mathop{C{{H}_{2}}BrC{{H}_{2}}C{{H}_{2}}Br}}\,\] ; \[\underset{\text{Tetra-methylene chloride}}{\mathop{Cl-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-Cl}}\,\] (iii) The tri-halogen derivatives are termed as halo-forms Example: \[\underset{\text{Chloroform}}{\mathop{CHC{{l}_{3}}}}\,\]; \[\underset{\text{Bromoform}}{\mathop{CHB{{r}_{3}}}}\,\]; \[\underset{I\text{odoform}}{\mathop{CH{{I}_{3}}}}\,\] (iv) In tetra-halogen derivatives all the four halogen atoms are attached to the same carbon atom in derivatives of methane. Example: \[\underset{\text{Carbon}\,\text{tetrachloride}}{\mathop{CC{{l}_{4}}}}\,\]; \[\underset{\text{Carbon}\,\text{tetrabromide}}{\mathop{CB{{r}_{4}}}}\,\] In other derivatives, the four halogen atoms are attached to different carbon atoms, e.g.,\[\underset{\begin{smallmatrix} \text{Acetylene tetrachloride or} \\ \text{1,1,2,2-tetrachloroethane} \end{smallmatrix}}{\mathop{\underset{CHC{{l}_{2}}}{\mathop{\underset{|\ \ \ \ \ \ \ \ \ \ \ }{\mathop{CHC{{l}_{2}}}}\,}}\,}}\,\] (3) The common and IUPAC names of some halogen derivatives are listed here.
(b) Vic-dihalides : In these derivatives, the halogen atoms are attached to adjacent (Vicinal) carbon atoms. These are also termed as alkylene halides. \[\underset{\text{Ethylene}\,\text{chloride}}{\mathop{C{{H}_{2}}Cl.C{{H}_{2}}Cl}}\,\] ; \[\underset{\text{Propylene}\,\text{chloride}}{\mathop{C{{H}_{3}}CHCl.C{{H}_{2}}Cl}}\,\] (c) a-w halides (Terminal dihalides): In these derivatives, the halogen atoms are attached to terminal carbon atoms. These are also called polymethylene halides. \[\underset{\text{Trimethylene bromide }}{\mathop{C{{H}_{2}}BrC{{H}_{2}}C{{H}_{2}}Br}}\,\] ; \[\underset{\text{Tetra-methylene chloride}}{\mathop{Cl-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-C{{H}_{2}}-Cl}}\,\] (iii) The tri-halogen derivatives are termed as halo-forms Example: \[\underset{\text{Chloroform}}{\mathop{CHC{{l}_{3}}}}\,\]; \[\underset{\text{Bromoform}}{\mathop{CHB{{r}_{3}}}}\,\]; \[\underset{I\text{odoform}}{\mathop{CH{{I}_{3}}}}\,\] (iv) In tetra-halogen derivatives all the four halogen atoms are attached to the same carbon atom in derivatives of methane. Example: \[\underset{\text{Carbon}\,\text{tetrachloride}}{\mathop{CC{{l}_{4}}}}\,\]; \[\underset{\text{Carbon}\,\text{tetrabromide}}{\mathop{CB{{r}_{4}}}}\,\] In other derivatives, the four halogen atoms are attached to different carbon atoms, e.g.,\[\underset{\begin{smallmatrix} \text{Acetylene tetrachloride or} \\ \text{1,1,2,2-tetrachloroethane} \end{smallmatrix}}{\mathop{\underset{CHC{{l}_{2}}}{\mathop{\underset{|\ \ \ \ \ \ \ \ \ \ \ }{\mathop{CHC{{l}_{2}}}}\,}}\,}}\,\] (3) The common and IUPAC names of some halogen derivatives are listed here.
| Formula of halogen derivatives | Common name | IUPAC name |
| \[C{{H}_{3}}Cl\] | Methyl chloride | Chloromethane |
| \[C{{H}_{3}}C{{H}_{2}}Br\] | Ethyl bromide | Bromoethane |
| \[C{{H}_{3}}CHBrC{{H}_{3}}\] | Isopropyl bromide | 2-Bromopropane |
| \[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{2}}Cl\] | n-Butyl chloride | 1-Chlorobutane |
| | Isobutyl bromide | 1-Bromo -2- methylpropane |
| \[C{{H}_{3}}-\overset{C{{H}_{3\,\,\,\,\,\,\,\,}}}{\mathop{\underset{Br\,\,\,\,\,\,\,\,\,\,\,}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,-C{{H}_{3}}}}\,}}\,\] | Tertiary butyl bromide | 2-Bromo ?2-methylpropane |
| Ethylidene chloride | 1,1-Dichloroethane | |
| \[C{{H}_{2}}Cl.C{{H}_{2}}Cl\] | Ethylene chloride | 1,2,-Dichloroethane |
| \[CHC{{l}_{3}}\] | Chloroform | Trichloromethane |
| \[CH{{I}_{3}}\] | Iodoform | Tri-iodomethane |
| \[CC{{l}_{4}}\] | Carbon tetrachloride | Tetrachloromethane |
You need to login to perform this action.
You will be redirected in
3 sec
