Organometallic Compounds
Category : JEE Main & Advanced
Organic compounds in which a metal atom is directly linked to carbon or organic compounds which contain at least one carbon-metal bond are called organometallic compounds.
Example : Methyl lithium \[(C{{H}_{3}}Li)\], Dialkyl zinc \[({{R}_{2}}Zn)\], Alkyl magnesium halide \[(R-Mg-X)\]
(1) Methyl lithium :
\[\underset{\text{Methyl}\,\text{iodide}}{\mathop{C{{H}_{3}}I}}\,+2Li\underset{-10{}^\circ C}{\mathop{\xrightarrow{\text{Ether}}}}\,\underset{\text{Methyl}\,\text{lithium}}{\mathop{C{{H}_{3}}Li}}\,+LiI\]
Chemical properties
(i) \[C{{H}_{3}}-Li+H\cdot OH\xrightarrow{{}}C{{H}_{4}}+LiOH\]
![]()
\[\xrightarrow{{{H}_{2}}O}C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}OH+LiOH\]
(iii) \[C{{H}_{3}}-Li+C{{O}_{2}}\xrightarrow{{}}C{{H}_{3}}\overset{O}{\mathop{-\overset{||}{\mathop{C}}\,-}}\,O-Li\]\[\xrightarrow{{{H}_{2}}O}C{{H}_{3}}COOH+LiOH\]
(iv) \[C{{H}_{3}}-Li+H\underset{H}{\mathop{-\underset{|}{\mathop{C}}\,=}}\,O\xrightarrow{{}}C{{H}_{3}}C{{H}_{2}}-O-Li\]\[\xrightarrow{{{H}_{2}}O}C{{H}_{3}}C{{H}_{2}}OH+LiOH\]
\[R-Li+C{{H}_{2}}=C{{H}_{2}}\xrightarrow{{}}R-C{{H}_{2}}-C{{H}_{2}}-Li\]
(2) Dialkyl zinc : First organometallic compound discovered by Frankland in 1849.
\[2RI+2Zn\underset{C{{O}_{2}}}{\mathop{\xrightarrow{Heat}}}\,2R-Zn-I\underset{C{{O}_{2}}}{\mathop{\xrightarrow{Heat}}}\,\underset{\text{Dialkyl}\,\text{zinc}}{\mathop{{{R}_{2}}Zn}}\,+Zn{{I}_{2}}\]
Chemical properties
Preparation of quaternary hydrocarbon :
\[{{(C{{H}_{3}})}_{3}}CCl+{{(C{{H}_{3}})}_{2}}Zn\xrightarrow{{}}\underset{\text{Neopentane}}{\mathop{{{(C{{H}_{3}})}_{4}}C}}\,+C{{H}_{3}}ZnCl\]
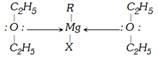
(3) Grignard reagent : Grignard reagent are prepared by the action of alkyl halide on dry burn magnesium in presence of alcohol free dry ether.
Dry ether dissolves the grignard reagent through solvation.
Grignard reagents are never isolated in free sate on account of their explosive nature.
For given alkyl radical the ease of formation of a grignard reagent is, Iodide > Bromide > Chloride Usually alkyl bromides are used.
For a given halogen, the ease of formation of grignard reagent is,\[C{{H}_{3}}X>{{C}_{2}}{{H}_{5}}X>{{C}_{3}}{{H}_{7}}X..........\]
Since tertiary alkyl iodides eliminate HI to form an alkene, tertiary alkyl chlorides are used in place of tertiary alkyl iodides.
The \[C-Mg\] bond in grignard reagent is some what covalent but highly polar.
![]()
The alkyl group acts as carbanion. The majority of reaction of grignard reagent fall into two groups:
(i) Double decomposition with compound containing active hydrogen atom or reactive halogen atom
\[RMgX+HOH\xrightarrow{{}}RH+Mg(OH)X\]
\[RMgX+{{D}_{2}}O\xrightarrow{{}}RD+Mg(OD)X\]
\[RMgX+R'OH\xrightarrow{{}}RH+Mg(OR')X\]
\[RMgX+R'N{{H}_{2}}\xrightarrow{{}}RH+Mg(R'NH)X\]
\[RMgX+R'I\xrightarrow{{}}R-R'+MgIX\]
\[RMgX+ClC{{H}_{2}}OR'\xrightarrow{{}}RC{{H}_{2}}OR'+MgClX\]
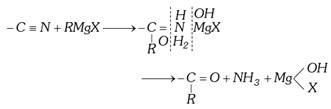
(ii) Addition reaction with compounds containing
![]()
![]()
You need to login to perform this action.
You will be redirected in
3 sec
