Properties Of Alkyl Halides
Category : JEE Main & Advanced
(1) Physical properties
(i) \[C{{H}_{3}}F,C{{H}_{3}}Cl,C{{H}_{3}}Br\] and \[{{C}_{2}}{{H}_{5}}Cl\] are gases at room temperature. The alkyl halides upto \[{{C}_{18}}\]are colourless liquids while higher members are colourless solids.
(ii) Alkyl halides are insoluble in water but soluble in organic solvents.
(iii) They burn on copper wire with green edged flame (Beilstein test for halogens).
(iv) Alkyl bromides and iodides are heavier than water. Alkyl chlorides and fluorides are lighter than water.
(v) Alkyl iodides become violet or brown in colour on exposure as they decompose in light.
\[2RI\xrightarrow{\text{Light}}R-R+{{I}_{2}}\]
(vi) For a given alkyl group, the boiling points of alkyl halides are in the order \[RI>RBr>RCl>RF\] and for a given halogen the boiling points of alkyl halides increase with the increase of the size of the alkyl group.
(vii) Alkyl halides are in general toxic compounds and bring unconsciousness when inhaled in large amounts.
(2) Chemical properties : The alkyl halides are highly reactive, the order of reactivity is,
Iodide > Bromide > Chloride (Nature of the halogen atom)
Tertiary > Secondary > Primary (Type of the halogen atom)
Amongst the primary alkyl halide, the order of reactivity is : \[C{{H}_{3}}X>{{C}_{2}}{{H}_{5}}X>{{C}_{3}}{{H}_{7}}X\], etc.
The high reactivity of alkyl halides can be explained in terms of the nature of \[C-X\] bond which is highly polarised covalent bond due to large difference in the electronegativities of carbon and halogen atoms. The halogen is far more electronegative than carbon and tends to pull the electrons away from carbon, i.e., halogen acquires a small negative charge and carbon a small positive charge.
\[-\overset{{{\delta }^{+}}}{\mathop{C}}\,-\overset{{{\delta }^{-}}}{\mathop{X}}\,\]
This polarity is responsible for reactions,
(i) Nucleophilic substitution reactions (ii) Elimination reactions
(i) Nucleophilic substitution (SN) reactions : The \[{{C}^{{{\delta }^{+}}}}\] site is susceptible to attack by nucleophiles (An electron rich species).
\[N{{u}^{-}}+R-X\xrightarrow{{}}Nu-R+{{X}^{-}}\]
\[R-X\underset{\text{Slow}}{\mathop{\xrightarrow{-{{X}^{-}}}}}\,{{R}^{+}}\underset{\text{Fast}}{\mathop{\xrightarrow{N{{u}^{-}}}}}\,R-Nu\] (\[{{S}_{{{N}^{1}}}}\]reaction)
\[N{{u}^{-}}+R-X\xrightarrow{\text{Slow}}\underset{\text{Transition}\,\text{state}}{\mathop{Nu.....R.....X}}\,\xrightarrow{\text{Fast}}Nu-R+{{X}^{-}}\] (\[{{S}_{{{N}^{2}}}}\] reaction)
Examples of \[{{S}_{N}}\] reactions,
(a) Hydrolysis :
\[\underset{\text{Alkyl}\,\text{halide}}{\mathop{RX}}\,+AgOH\xrightarrow{{}}\underset{\text{Alcohol}}{\mathop{ROH}}\,+AgX\]
\[RX+KOH(aq)\xrightarrow{{}}ROH+KX\]
\[\underset{\text{Ethylene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,\xrightarrow{HBr}\underset{\text{Ethyl bromide}}{\mathop{C{{H}_{3}}C{{H}_{2}}Br}}\,\xrightarrow{AgOH}\underset{\text{Ethyl alcohol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,\]
(b) Reaction with alkoxides or dry silver oxide :
\[RX+NaOR'\xrightarrow{Heat}\underset{\text{Unsym}\text{.}\,\text{ether}}{\mathop{ROR'}}\,+NaX\]
\[2RX+A{{g}_{2}}O\xrightarrow{{}}\underset{\text{Sym}\text{.}\,\text{ether}}{\mathop{R-O-R}}\,+2AgX\]
(c) Reaction with sodium or potassium hydrogen sulphide :
\[RX+\underset{\text{sulphide}}{\mathop{\underset{\text{Sodium}\,\text{hydrogen}\,}{\mathop{NaSH}}\,}}\,\xrightarrow{{}}\underset{\begin{smallmatrix} \text{or}\,\text{Alkanethiol} \\ \text{or Alkyl mercaptan} \end{smallmatrix}}{\mathop{\underset{\text{Thioalcohol }}{\mathop{RSH}}\,}}\,+NaX\]
(d) Reaction with alcoholic potassium cyanide and silver cyanide :
\[RX+KCN\xrightarrow{Alcohol}\underset{\begin{smallmatrix} \text{Alkyl}\,\text{cyanide}\,\text{or} \\ \text{Alkane nitrile} \end{smallmatrix}}{\mathop{RCN}}\,+KX\]
(e) Reaction with potassium nitrite or silver nitrite :
\[RX+K-O-N=O\xrightarrow{\Delta }\underset{\text{Alkyl}\,\text{nitrite}}{\mathop{R-O-N=O}}\,+KX\]
![]()
(f) Reaction with ammonia :
\[{{C}_{2}}{{H}_{5}}Br+H-N{{H}_{2}}\xrightarrow{{}}\underset{\text{Ethylamine(p}\text{.)}}{\mathop{{{C}_{2}}{{H}_{5}}N{{H}_{2}}}}\,+HBr\]
\[{{C}_{2}}{{H}_{5}}N{{H}_{2}}+Br{{C}_{2}}{{H}_{5}}\xrightarrow{{}}\underset{\text{Diethylamine(sec}\text{.)}}{\mathop{{{C}_{2}}{{H}_{5}}NH{{C}_{2}}{{H}_{5}}}}\,+HBr\]
\[{{({{C}_{2}}{{H}_{5}})}_{2}}NH+Br{{C}_{2}}{{H}_{5}}\xrightarrow{{}}\underset{\text{Triethylamine(tert}\text{.)}}{\mathop{{{({{C}_{2}}{{H}_{5}})}_{3}}N}}\,+HBr\]
\[{{({{C}_{2}}{{H}_{5}})}_{3}}N+Br{{C}_{2}}{{H}_{5}}\xrightarrow{{}}\underset{\begin{smallmatrix} \text{Tetraethyl}\,\text{ammonium} \\ \text{bromide(Quaternary)} \end{smallmatrix}}{\mathop{{{({{C}_{2}}{{H}_{5}})}_{4}}\overset{+\,\,\,\,\,-}{\mathop{NBr}}\,}}\,\]
(g) Reaction with silver salts of fatty acids :
\[R'COOAg+XR\xrightarrow{{}}\underset{\text{Ester}}{\mathop{R'COOR}}\,\,+AgX\]
(h) Reaction with sodium acetylide :
\[RX+\underset{\text{Sodium acetylide}}{\mathop{NaC\equiv CH}}\,\xrightarrow{{}}\underset{\text{Alkyne}}{\mathop{R-C\equiv CH}}\,+NaX\]
(i) Reaction with sodium or potassium sulphide :
\[2RX+N{{a}_{2}}S\xrightarrow{{}}\underset{\text{Thioether}}{\mathop{R-S-R}}\,+2NaX\]
Thioethers can also be obtained by
\[RX-NaSR'\xrightarrow{{}}R-S-R'+NaX\]
\[{{C}_{2}}{{H}_{5}}Br+NaSC{{H}_{3}}\xrightarrow{{}}\underset{\text{Ethyl methyl thioether}}{\mathop{{{C}_{2}}{{H}_{5}}-S-C{{H}_{3}}}}\,+NaBr\]
(j) Reaction with halides :
\[\underset{\text{Alkyl}\,\text{chloride}}{\mathop{RCl}}\,+NaBr\xrightarrow{{}}\underset{\text{Alkyl bromide}}{\mathop{RBr}}\,\xrightarrow{NaI}\underset{\text{Alkyl iodide}}{\mathop{RI}}\,\]
(ii) Elimination reactions : The positive charge on carbon is propagated to the neighbouring carbon atoms by inductive effect. When approached by a strongest base (B), it tends to lose a proton usually from the \[\beta -\]carbon atom. Such reactions are termed elimination reactions. They are also \[{{E}_{1}}\] and \[{{E}_{2}}\] reactions.
\[{{E}_{1}}\] reaction : \[R-\underset{H}{\overset{\text{H}}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-\underset{X}{\overset{H}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,-H\underset{-{{X}^{-}}}{\mathop{\xrightarrow{\text{Slow}}}}\,R-\overset{{{B}^{-}}}{\mathop{\underset{H}{\overset{\overset{.\,.}{\mathop{H}}\,}{\mathop{\underset{|}{\overset{|}{\mathop{C}}}\,}}}\,}}\,-\overset{H}{\mathop{\underset{+}{\mathop{\overset{|}{\mathop{C}}\,}}\,}}\,-H\]\[\xrightarrow{\text{Fast}}R-\underset{H}{\mathop{\underset{|}{\mathop{C}}\,}}\,=\overset{H}{\mathop{\overset{|}{\mathop{C}}\,}}\,-H+B-H\]
\[{{E}_{2}}\] Reaction :
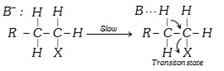
\[\xrightarrow{\text{Fast}}R-\underset{H}{\mathop{\underset{|}{\mathop{C}}\,}}\,=\overset{\text{H}}{\mathop{\overset{|}{\mathop{C}}\,}}\,-H+B-H+{{X}^{-}}\]
As the above reactions involve leaving of \[{{X}^{-}},\] the reactivity of alkyl halides (Same alkyl group, different halogens) should be limited with \[C-X\] bond strength.
Type of bond
Bond strength (kcal/mol)

The breaking of the bond becomes more and more difficult and thus, the reactivity decrease.
The order of reactivity (Tertiary > Secondary > Primary) is due to +I effect of the alkyl groups which increases the polarity of \[C-X\] bond.
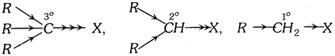
The primary alkyl halides undergo reactions either by \[{{S}_{{{N}^{2}}}}\]or \[{{E}_{2}}\] mechanisms which involve the formation of transition state. The bulky groups cause steric hinderance in the formation of transition state. Therefore, higher homologues are less reactive than lower homologues. \[C{{H}_{3}}X>{{C}_{2}}{{H}_{5}}X>{{C}_{3}}{{H}_{7}}X\], etc.
Example of elimination reaction
(a) Dehydrohalogenation :
\[{{C}_{n}}{{H}_{2n+1}}X+\underset{\text{(Alcoholic)}}{\mathop{KOH}}\,\xrightarrow{{}}\underset{\text{Alkene}}{\mathop{{{C}_{n}}{{H}_{2n}}}}\,+KX+{{H}_{2}}O\]
In this reactions, ether is a by-product as potassium ethoxide is always present in small quantity.
\[{{C}_{2}}{{H}_{5}}Br+KO{{C}_{2}}{{H}_{5}}\xrightarrow{{}}{{C}_{2}}{{H}_{5}}-O-{{C}_{2}}{{H}_{5}}+KBr\]
(b) Action of heat :
\[RC{{H}_{2}}C{{H}_{2}}X\xrightarrow{300{}^\circ C}\underset{\text{Alkene}}{\mathop{RCH=C{{H}_{2}}}}\,+HX\]
\[{{C}_{2}}{{H}_{5}}Br\xrightarrow{300{}^\circ C}\underset{\text{Ethene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,+HBr\]
The decomposition follows the following order,
Iodide > Bromide > Chloride (When same alkyl group is present) and
Tertiary > Secondary > Primary (When same halogen is present).
(iii) Miscellaneous reactions
(a) Reduction : Alkyl halides are reduced with nascent hydrogen obtained by \[Zn/HCl\] or sodium and alcohol or Zn/Cu couple or \[LiAl{{H}_{4}}\].
\[RX+2H\xrightarrow{{}}R-H+HX\]
Reaction is used for the preparation of pure alkanes
(b) Wurtz reaction : An ether solution of an alkyl halide (Preferably bromide or iodide) gives an alkane when heated with metallic sodium.
\[2RX+2Na\xrightarrow{{}}R-R+2NaX\]
(c) Reaction with magnesium : Alkyl halides form Grignard reagent when treated with dry magnesium powder in dry ether.
\[RX+\underset{\text{(Powder )}}{\mathop{Mg}}\,\xrightarrow{\text{Dry}\,\text{ether}}\underset{\text{Grignard reagent}}{\mathop{R-Mg-X}}\,\]
Grignard reagents are used for making a very large number of organic compounds.
(d) Reaction with other metals : Organometallic compounds are formed.
\[2{{C}_{2}}{{H}_{5}}Br+2Zn\underset{\text{Heat}}{\mathop{\xrightarrow{\text{Ether}}}}\,{{({{C}_{2}}{{H}_{5}})}_{2}}Zn+ZnB{{r}_{2}}\]
\[4{{C}_{2}}{{H}_{5}}Br+4Pb(Na)\xrightarrow{{}}{{({{C}_{2}}{{H}_{5}})}_{4}}Pb+4NaBr+3Pb\]
\[RX+2Li\xrightarrow{\text{Ether}}R-Li+LiX\];
\[\underset{\text{Ethyl}\,\text{bromide}}{\mathop{{{C}_{2}}{{H}_{5}}Br}}\,+2Li\xrightarrow{{}}{{C}_{2}}{{H}_{5}}-Li+LiBr\]
Alkyl lithiums are similar in properties with Grignard reagents. These are reactive reagents also.
(e) Friedel-Craft's reaction :
\[\underset{\text{Benzene}}{\mathop{{{C}_{6}}{{H}_{6}}}}\,+RCl\xrightarrow{AlC{{l}_{3}}}\underset{\text{Alkyl}\,\text{benzene}}{\mathop{{{C}_{6}}{{H}_{5}}R}}\,+HCl\]
\[{{C}_{6}}{{H}_{6}}+{{C}_{2}}{{H}_{5}}Br\xrightarrow{AlB{{r}_{3}}}{{C}_{6}}{{H}_{5}}{{C}_{2}}{{H}_{5}}+HBr\]
(f) Substitution (Halogenation) : Alkyl halides undergo further halogenation in presence of sunlight, heat energy or peroxide.
\[{{C}_{2}}{{H}_{5}}Br\underset{hv}{\mathop{\xrightarrow{B{{r}_{2}}}}}\,{{C}_{2}}{{H}_{4}}B{{r}_{2}}\underset{hv}{\mathop{\xrightarrow{B{{r}_{2}}}}}\,{{C}_{2}}{{H}_{3}}B{{r}_{3}}.....\]
You need to login to perform this action.
You will be redirected in
3 sec
