Unsaturated Halides (Halo-alkene)
Category : JEE Main & Advanced
Vinyl chloride or chloroethene, \[C{{H}_{\mathbf{2}}}=CHCl\]
(1) Synthesis : Vinyl chloride can be synthesised by a number of methods described below:
(i) From ethylene chloride :
\[\underset{\text{Ethylene chloride}}{\mathop{\underset{C{{H}_{2}}Cl}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}Cl}}\,}}\,+Alc.\,\,\,KOH\,\,\xrightarrow{{}}\ \ \underset{\text{Vinyl chloride}}{\mathop{\underset{C{{H}_{2}}\,\,\,\,\,}{\mathop{\underset{|\,|}{\mathop{C}}\,HCl}}\,}}\,+KCl+{{H}_{2}}O\]
\[\underset{C{{H}_{2}}Cl}{\mathop{\underset{|}{\mathop{C}}\,{{H}_{2}}Cl}}\,\ \xrightarrow[600-{{650}^{o}}C]{\Delta }\ \,\,\underset{C{{H}_{2}}\,\,\,}{\mathop{\underset{|\,|}{\mathop{C}}\,HCl}}\,+HCl\]
(ii) From ethylene :
\[C{{H}_{2}}=C{{H}_{2}}+C{{l}_{2}}\xrightarrow{500{}^\circ C}\underset{\text{Vinyl chloride}}{\mathop{C{{H}_{2}}=CHCl}}\,\]
(iii) From acetylene :
\[CH\equiv CH+HCl\underset{70{}^\circ C}{\mathop{\xrightarrow{HgC{{l}_{2}}}}}\,\underset{\text{Vinyl chloride}}{\mathop{C{{H}_{2}}=CHCl}}\,\]
(2) Properties : It is a colourless gas at room temperature. Its boiling point is \[-{{13}^{o}}C\]. The halogen atom in vinyl chloride is not reactive as in other alkyl halides. However, \[C=C\] bond of vinyl chloride gives the usual addition reactions.
The non-reactivity of chlorine atom is due to resonance stabilization. The lone pair on chlorine can participate in delocalization (Resonance) to give two canonical structures.
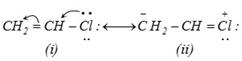
The following two effects are observed due to resonance stabilization.
(i) Carbon-chlorine bond in vinyl chloride has some double bond character and is, therefore, stronger than a pure single bond.
(ii) Carbon atom is \[s{{p}^{2}}\] hybridized and \[C-Cl\] bond length is shorter \[(1.69{\AA})\] and the bond is stronger than in alkyl halides (1.80Å) due to \[s{{p}^{3}}\] hybridization of the carbon atom.
Addition reactions
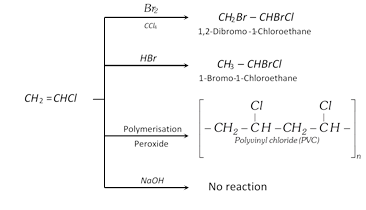
(3) Uses : The main use of vinyl chloride is in the manufacture of polyvinyl chloride (PVC) plastic which is employed these days for making synthetic leather goods, rain coats, pipes, floor tiles, gramophone records, packaging materials, etc.
You need to login to perform this action.
You will be redirected in
3 sec
