Alkenes
Category : JEE Main & Advanced
These are the acyclic hydrocarbon in which carbon-carbon contain double bond. These are also known as olefins, because lower alkene react with halogens to form oily substances. General formula is \[{{C}_{n}}{{H}_{2n}}\]. Examples, \[{{C}_{2}}{{H}_{4}},\,{{C}_{3}}{{H}_{6}},\,{{C}_{4}}{{H}_{8}}\].
(1) Preparation methods
(i) From Alkynes :
\[R-C\equiv C-R+{{H}_{2}}\underset{Pd.\,BaS{{O}_{4}}}{\mathop{\xrightarrow{\text{Lindlar }\!\!'\!\!\text{ s}\,\text{Catalyst}}}}\,R-\overset{H}{\mathop{\overset{|}{\mathop{C}}\,}}\,=\underset{H}{\mathop{\underset{|}{\mathop{C}}\,}}\,-R\]
(ii) From mono halides :
\[R-\overset{H}{\mathop{\overset{|}{\mathop{\underset{H}{\mathop{\underset{|}{\mathop{C}}\,}}\,}}\,}}\,-\overset{H}{\mathop{\overset{|}{\mathop{\underset{X}{\mathop{\underset{|}{\mathop{C}}\,}}\,}}\,}}\,-H+Alc.\,KOH\xrightarrow[-HX]{}\underset{\text{Alkene}}{\mathop{R-\underset{H}{\mathop{\underset{|}{\mathop{C}}\,}}\,=}}\,\overset{H}{\mathop{\overset{|}{\mathop{C}}\,}}\,-H\]
(iii) From dihalides
(a) From Gem dihalides
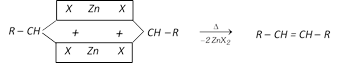
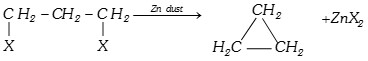
(b) From vicinal dihalides :
\[R-\overset{H}{\mathop{\overset{|}{\mathop{\underset{X}{\mathop{\underset{|}{\mathop{C}}\,}}\,}}\,}}\,-\overset{H}{\mathop{\overset{|}{\mathop{\underset{X}{\mathop{\underset{|}{\mathop{C}}\,}}\,}}\,}}\,-H+Zn\,dust\underset{{{300}^{o}}C}{\mathop{\xrightarrow{\Delta }}}\,R-\overset{H}{\mathop{\overset{|}{\mathop{C}}\,}}\,=\overset{H}{\mathop{\overset{|}{\mathop{C}}\,}}\,-H+Zn{{X}_{2}}\]
(iv) By action of on vicinal dihalide :
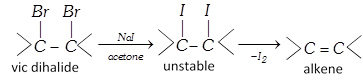
(v) From alcohols [Laboratory method] :
\[\underset{\text{Ethyl alcohol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,\underset{443\,K}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}}\,or\,{{H}_{3}}P{{O}_{4}}}}}\,\underset{\text{Ethene}}{\mathop{C{{H}_{2}}}}\,=C{{H}_{2}}+{{H}_{2}}O\]
(vi) Kolbe’s reaction :
\[\underset{\text{Potassium succinate}}{\mathop{\begin{array}{*{35}{l}} C{{H}_{2}}COOK \\ \,| \\ C{{H}_{2}}COOK \\ \end{array}}}\,+2{{H}_{2}}O\xrightarrow{\text{Electrolysis}}\underset{\text{Ethene}}{\mathop{\begin{array}{*{35}{l}} C{{H}_{2}} \\ \,| \\ C{{H}_{2}} \\ \end{array}}}\,+2C{{O}_{2}}+{{H}_{2}}+2KOH\]
(vii) From esters [Pyrolysis of ester] :
\[C{{H}_{3}}-CO-\underset{C{{H}_{2}}-C{{H}_{2}}}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|\,\,\,\,\,\,\,}{\mathop{O\,\,\,\,\,\,\,\,\,\,\,\,H\,\,\,\,\,\,}}\,}}\,\underset{liq.\,{{N}_{2}}}{\mathop{\begin{align} & \\ & \xrightarrow{\text{Glass}\,\text{wool}\,{{450}^{o}}} \\ \end{align}}}\,\underset{C{{H}_{2}}=C{{H}_{2}}}{\mathop{\underset{+}{\mathop{C{{H}_{3}}-COOH}}\,}}\,\]
(viii) Pyrolysis of quaternary ammonium compounds :
\[\underset{\underset{\text{hydroxide}}{\mathop{\text{Tetraethyl}\,\text{ammonium}}}\,}{\mathop{{{({{C}_{2}}{{H}_{5}})}_{4}}\overset{+}{\mathop{N}}\,\overset{-}{\mathop{OH}}\,}}\,\xrightarrow{heat}\underset{\underset{\text{(Tert}\text{.}\,\text{amine)}}{\mathop{\text{Triethylamine}}}\,}{\mathop{{{({{C}_{2}}{{H}_{5}})}_{3}}N}}\,+\underset{\text{Ethene}}{\mathop{{{C}_{2}}{{H}_{4}}}}\,+{{H}_{2}}O\]
(ix) Action of copper alkyl on vinyl chloride :
\[\underset{\text{Vinyl}\,\text{chloride}}{\mathop{{{H}_{2}}C=CHCl}}\,\xrightarrow{Cu{{R}_{2}}}{{H}_{2}}C=CHR\]
(x) By Grignard reagents :
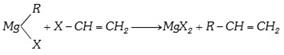
(xi) The wittig reaction :
\[{{(Ph)}_{3}}P=C{{H}_{2}}+\underset{O\,\,\,}{\mathop{\underset{|\,|\,\,\,\,}{\mathop{CH}}\,}}\,-R\xrightarrow{{}}{{(Ph)}_{3}}P=O+R-\underset{C{{H}_{2}}}{\mathop{\underset{|\,|\,\,\,\,\,\,}{\mathop{CH\,}}\,}}\,\]
\[{{(Ph)}_{3}}P=CH-R+\overset{O\,\,\,}{\mathop{\overset{|\,|\,\,\,\,}{\mathop{CH}}\,}}\,-R\xrightarrow{{}}{{(Ph)}_{3}}P=O+R-CH=CH-R\]
(xii) From \[\beta \]bromo ether [Boord synthesis]
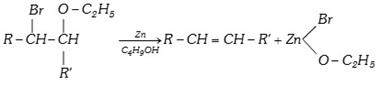
(2) Physical Properties
(i) Alkenes are colourless and odourless.
(ii) These are insoluble in water and soluble in organic solvents.
(iii) Physical state
\[{{C}_{1}}-{{C}_{4}}\xrightarrow{{}}\] gas
\[{{C}_{4}}-{{C}_{16}}\xrightarrow{{}}\] liquid
\[>\,\,\,{{C}_{17}}\xrightarrow{{}}\] solid wax
(iv) B.P. and M.P. decreases with increasing branches in alkene.
(v) The melting points of cis isomers are lower than trans isomers because cis isomer is less symmetrical than trans. Thus trans packs more tightly in the crystal lattice and hence has a higher melting point.
(vi) The boiling points of cis isomers are higher than trans isomers because cis-alkenes has greater polarity (Dipole moment) than trans one.
(vii) These are lighter than water.
(viii) Dipole moment : Alkenes are weakly polar. The, \[\pi -\]electron’s of the double bond. Can be easily polarized. Therefore, their dipole moments are higher than those of alkanes.
(3) Chemical properties
(i) Francis experiment : According to Francis electrophile first attacks on olefinic bond.
(ii) Reaction with hydrogen :
\[R-\overset{H\,\,\,\,H}{\mathop{\overset{|\,\,\,\,\,\,\,\,|}{\mathop{C=C}}\,}}\,-R+{{H}_{2}}\xrightarrow{Ni}R-\underset{H\,\,\,H}{\mathop{\underset{|\,\,\,\,\,\,\,|}{\mathop{\overset{H\,\,\,H}{\mathop{\overset{|\,\,\,\,\,\,\,|}{\mathop{C-C}}\,}}\,}}\,}}\,-R\]
(iii) Reduction of alkene via hydroboration : Alkene can be converted into alkane by protolysis
\[RCH=C{{H}_{2}}\xrightarrow{H-B{{H}_{2}}}{{(R-C{{H}_{2}}-C{{H}_{2}})}_{3}}B\]
\[\xrightarrow{{{H}^{+}}/{{H}_{2}}O}R-C{{H}_{2}}-C{{H}_{3}}\]
Hydroboration : Alkene give addition reaction with diborane which called hydroboration. In this reaction formed trialkylborane, Which is very important and used for synthesis of different organic compound
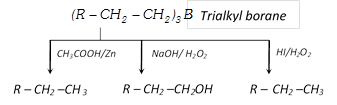
The overall result of the above reaction appears to be antimarkownikoff’s addition of water to a double bond.
(iv) By treatment with \[\mathbf{AgN}{{\mathbf{O}}_{\mathbf{3}}}\mathbf{+NaOH}\] : This reaction gives coupling
\[6C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-\overset{C{{H}_{3}}}{\mathop{\overset{|\,\,\,\,\,\,\,\,}{\mathop{C\,\,=\,\,\,}}\,}}\,C{{H}_{2}}\xrightarrow{{{B}_{2}}{{H}_{6}}}\]
\[2{{[C{{H}_{3}}-{{(C{{H}_{2}})}_{2}}-\underset{H\,\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,}{\mathop{\overset{C{{H}_{3}}}{\mathop{\overset{|\,\,\,\,\,\,\,\,}{\mathop{C\,\,-\,\,}}\,}}\,}}\,}}\,C{{H}_{2}}]}_{3}}B\xrightarrow{Ag/N{{O}_{3}}NaOH}\]
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-\underset{H\,\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,}{\mathop{\overset{C{{H}_{3}}}{\mathop{\overset{|\,\,\,\,\,\,\,\,}{\mathop{C\,\,-\,\,}}\,}}\,}}\,}}\,C{{H}_{2}}-C{{H}_{2}}-\underset{H\,\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,}{\mathop{\overset{C{{H}_{3}}}{\mathop{\overset{|\,\,\,\,\,\,\,\,}{\mathop{C\,\,-\,\,}}\,}}\,}}\,}}\,C{{H}_{2}}-C{{H}_{2}}-C{{H}_{3}}\]
(v) Birch reduction : This reaction is believed to proceed via anionic free radical mechanism.
\[R-CH=C{{H}_{2}}\underset{+{{e}^{-}}}{\mathop{\xrightarrow{Na}}}\,R-C\overset{-}{\mathop{H}}\,-C{{\overset{-}{\mathop{H}}\,}_{2}}\xrightarrow{Et-O-H}\]\[R-CH-C{{H}_{3}}\]
\[\underset{+{{e}^{-}}}{\mathop{\xrightarrow{Na}}}\,R-\overset{-}{\mathop{\underset{{}}{\mathop{C}}\,}}\,H-C{{H}_{3}}\xrightarrow{Et.-O-H}R-C{{H}_{2}}-C{{H}_{3}}\]
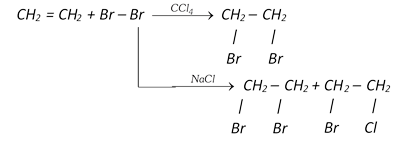
(vi) Halogenation
\[\underset{\text{Propene}}{\mathop{C{{H}_{3}}CH=C{{H}_{2}}}}\,+C{{l}_{2}}\xrightarrow{{{500}^{o}}C}\underset{\text{or 3-Chloro-1-propene}}{\mathop{\underset{\text{Allyl}\,\text{chloride}}{\mathop{ClC{{H}_{2}}-CH=C{{H}_{2}}}}\,}}\,+HCl\]
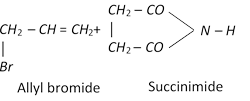
\[R-\overset{H\,\,\,\,H}{\mathop{\overset{|\,\,\,\,\,\,\,\,|}{\mathop{C=C}}\,}}\,-H+X-X\xrightarrow{CC{{l}_{4}}}\underset{\text{Vicinal dihalide}}{\mathop{R-\underset{X\,\,\,X}{\mathop{\underset{|\,\,\,\,\,\,\,|}{\mathop{\overset{H\,\,\,H}{\mathop{\overset{|\,\,\,\,\,\,\,|}{\mathop{C-C}}\,}}\,}}\,}}\,-H}}\,\]
Reactivity of halogen is \[{{F}_{2}}\,\,>\,\,\,C{{l}_{2}}\,\,>\,\,\,B{{r}_{2}}\,\,\,>\,\,\,{{I}_{2}}\]
(vii) Reaction with HX [Hydrohalogenation]
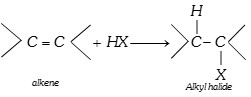
According to markownikoff’s rule and kharasch effect.
\[C{{H}_{3}}-CH=C{{H}_{2}}+HBr\xrightarrow{{}}C{{H}_{3}}-\overset{H\,\,\,\,H}{\mathop{\overset{|\,\,\,\,\,\,\,\,|}{\mathop{\underset{Br\,\,\,H}{\mathop{\underset{|\,\,\,\,\,\,\,\,|}{\mathop{C-C}}\,}}\,}}\,}}\,-H\]
According to Anti Markownikoff rule (Based on F.R.M.)
\[C{{H}_{3}}-CH=C{{H}_{2}}+HBr\xrightarrow{\text{Peroxide}}\]
\[\underset{(\text{minor)}}{\mathop{C{{H}_{3}}-\overset{H\,\,\,\,H}{\mathop{\overset{|\,\,\,\,\,\,\,\,|}{\mathop{\underset{Br\,\,\,H}{\mathop{\underset{|\,\,\,\,\,\,\,\,|}{\mathop{C-C}}\,}}\,}}\,}}\,-H}}\,\,+\,\underset{\text{(major)}}{\mathop{C{{H}_{3}}-\overset{H\,\,\,\,H}{\mathop{\overset{|\,\,\,\,\,\,\,\,|}{\mathop{\underset{H\,\,\,Br}{\mathop{\underset{|\,\,\,\,\,\,\,\,|}{\mathop{C-C}}\,}}\,}}\,}}\,-H}}\,\]
(viii) Reaction with hypohalous acids :
\[\underset{\text{Ethylene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,+H\overset{-}{\mathop{O}}\,\overset{+}{\mathop{Cl}}\,\xrightarrow{{}}\underset{\text{Ethylene chlorohydrin}}{\mathop{C{{H}_{2}}OH.C{{H}_{2}}Cl}}\,\]
(ix) Reaction with sulphuric acid :
\[\underset{\text{Ethylene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,+{{H}^{+}}HSO_{4}^{-}\xrightarrow{{}}\underset{\text{Ethyl hydrogen sulphate}}{\mathop{C{{H}_{3}}C{{H}_{2}}HS{{O}_{4}}}}\,\]
\[C{{H}_{3}}C{{H}_{2}}HS{{O}_{4}}\xrightarrow{{}}C{{H}_{2}}=C{{H}_{2}}+{{H}_{2}}S{{O}_{4}}\]
(x) Reaction with nitrosyl chloride
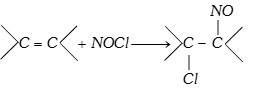
(\[NOCl\] is called Tillden reagent)
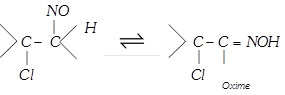
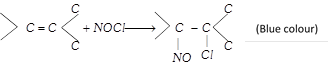
(xi) Oxidation : With alkaline \[KMn{{O}_{4}}\] [Bayer’s reagent] : This reaction is used as a test of unsaturation.
\[R-\overset{\,H\,\,\,\,H}{\mathop{\overset{\,|\,\,\,\,\,\,\,\,|}{\mathop{C=C}}\,}}\,-H+[O]+H-OH\underset{-OH}{\mathop{\xrightarrow{Alk\,KMn{{O}_{4}}}}}\,\underset{\text{glycol}}{\mathop{R\overset{H\,\,\,\,\,\,H}{\mathop{\overset{|\,\,\,\,\,\,\,\,\,|}{\mathop{\underset{HO\,\,\,\,OH}{\mathop{\underset{\,\,|\,\,\,\,\,\,\,\,|}{\mathop{-C-C}}\,-}}\,}}\,}}\,H}}\,\]
With acidic \[KMn{{O}_{4}}\] :
\[R-\overset{H}{\mathop{\overset{|}{\mathop{C}}\,}}\,=\overset{H}{\mathop{\overset{|}{\mathop{C}}\,}}\,-H+[O]\underset{KMn{{O}_{4}}}{\mathop{\xrightarrow{acidic}}}\,R-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-O-H+C{{O}_{2}}+{{H}_{2}}O\]
(xii) Hydroxylation
(a) Using per oxy acid :
\[\underset{\text{2-Butene}}{\mathop{\overset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{{H}_{3}}}{\mathop{\overset{\,\,\,\,\,\,\,\,\,|}{\mathop{\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,C{{H}_{3}}}{\mathop{\underset{\,\,\,\,\,\,\,\,\,|}{\mathop{\underset{H-C}{\mathop{\underset{\,\,\,\,\,\,\,\,\,||}{\mathop{H-C}}\,}}\,}}\,}}\,}}\,}}\,}}\,\underset{or\,HC{{O}_{3}}H}{\mathop{\xrightarrow{{{H}_{2}}{{O}_{2}},\,HCOOH}}}\,\underset{\text{Trans (racemic)}}{\mathop{\overset{\,\,\,\,\,\,\,\,\,C{{H}_{3}}}{\mathop{\overset{\,\,\,\,|}{\mathop{\underset{\,\,\,\,\,\,\,\,\,C{{H}_{3}}}{\mathop{\underset{\,\,\,\,|}{\mathop{\underset{HO-C-H}{\mathop{\underset{\,\,\,\,|}{\mathop{\,\,\,\,\,\,\,\,H-C-OH}}\,}}\,}}\,}}\,}}\,}}\,}}\,\]
(b) Hydroxylation by \[Os{{O}_{4}}\]:
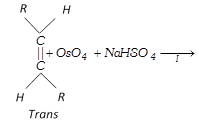
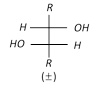
\[R-CH=CH-R\underset{or\,C{{H}_{3}}C{{O}_{3}}H}{\mathop{\xrightarrow{{{C}_{6}}{{H}_{5}}C{{O}_{3}}H}}}\,R-\underset{OH}{\mathop{\underset{|\,\,\,\,\,}{\mathop{CH}}\,}}\,-\underset{OH}{\mathop{\underset{|\,\,\,\,\,}{\mathop{CH}}\,}}\,-R\xrightarrow{-{{H}_{2}}O}\] \[\underset{\text{ }\!\![\!\!\text{ Oxirane }\!\!]\!\!\text{ }}{\mathop{R-\underset{O\,\,\,}{\mathop{\underset{{}}{\mathop{CH-CH}}\,}}\,-R}}\,\]
(xiii) Combustion : \[{{C}_{n}}{{H}_{2n}}+\frac{3n}{2}{{O}_{2}}\xrightarrow{{}}nC{{O}_{2}}+n{{H}_{2}}O\]
They burn with luminous flame and form explosive mixture with air or oxygen.
(xiv) Ozonolysis
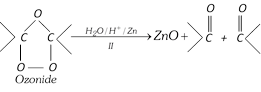
(xv) Oxy – mercuration demercuration : With mercuric acetate (in THF), followed by reduction with \[NaB{{H}_{4}}/NaOH\] is also an example of hydration of alkene according to markownikoff’s rule.
\[\underset{\text{3,3-dimethyl-1-butene}}{\mathop{{{(C{{H}_{3}})}_{3}}C-CH=C{{H}_{2}}}}\,+\underset{\text{Mercuric acetate}}{\mathop{{{(C{{H}_{3}}COO)}_{2}}Hg}}\,\xrightarrow{{}}\]
\[{{(C{{H}_{3}})}_{3}}C-\underset{OCOC{{H}_{3}}}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{CH-C{{H}_{2}}}}\,}}\,-Hg\underset{THF}{\mathop{\xrightarrow{NaB{{H}_{4}}/NaOH}}}\,\underset{3,\,3-\text{Dimethyl}-\text{2}-\text{butanol}}{\mathop{{{(C{{H}_{3}})}_{3}}C-\underset{OH}{\mathop{\underset{|\,\,\,\,\,}{\mathop{CH}}\,}}\,-C{{H}_{3}}}}\,\]
(xvi) Epoxidation
(a) By \[{{O}_{2}}/Ag\] :
![]()
(b) Epoxidation by performic acid or perbenzoic acid :
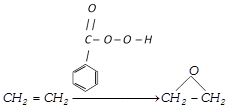
![]()
(xvii) Hydroboration
\[3R-CH=C{{H}_{2}}+B{{H}_{3}}\xrightarrow{{}}\underset{\text{Tri alkyl borane}}{\mathop{{{(R-C{{H}_{2}}-C{{H}_{2}})}_{3}}B}}\,\xrightarrow{{{H}_{2}}{{O}_{2}}/O{{H}^{-}}}R-C{{H}_{2}}-C{{H}_{2}}-OH+B{{(OH)}_{3}}\]
\[R-C{{H}_{2}}-C{{H}_{2}}-OH+B{{(OH)}_{3}}\]
(Anti markownikoff’s rule)
(xviii) Hydroformylation :
\[R-CH=C{{H}_{2}}+CO+{{H}_{2}}\xrightarrow{CoH{{(CO)}_{4}}}\underset{\,\,\,\,\,\,\,H}{\overset{\,\,\,\,\,\,\,\,\,H}{\mathop{\underset{\,\,\,\,\,\,\,\,|}{\overset{\,\,\,\,\,\,\,\,\,|}{\mathop{R-C}}}\,}}}\,-\underset{H\,\,\,\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,}{\mathop{\underset{C\,=\,O\,}{\mathop{\overset{H\,\,\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,}{\mathop{\overset{|\,\,\,\,\,\,\,\,}{\mathop{C-H}}\,}}\,}}\,}}\,}}\,}}\,\]
\[R-CH=C{{H}_{2}}+CO+{{H}_{2}}O\xrightarrow{CoH{{(CO)}_{4}}}R-C{{H}_{2}}-\underset{COOH}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}\,\,\,\,}}\,}}\,\]
(xix) Addition of formaldehyde
\[{{H}_{2}}C=O+\overset{\oplus }{\mathop{H}}\,\xrightarrow{{}}[{{H}_{2}}C=\overset{\oplus }{\mathop{O}}\,H\overset{{}}{\longleftrightarrow}{{H}_{2}}\overset{\oplus }{\mathop{C}}\,-OH]\]
\[\xrightarrow{R-CH=C{{H}_{2}}}R-\overset{\oplus }{\mathop{C}}\,H-C{{H}_{2}}-C{{H}_{2}}-OH\frac{\,\,\,\,\,HOH\,\,\,\,}{-{{H}^{+}}}\]
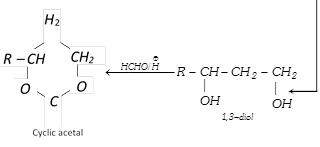
(xx) Polymerisation
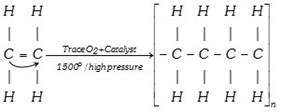
(xxi) Isomerisation :
\[C{{H}_{3}}-C{{H}_{2}}-C{{H}_{2}}-CH=C{{H}_{2}}\]
\[\]\[C{{H}_{3}}-C{{H}_{2}}-CH=CH-C{{H}_{3}}\]
The mechanism proceeds via carbocation.
(xxii) Addition of \[HN{{O}_{3}}\] :
\[\underset{\text{Ethene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,+HO-N{{O}_{2}}\xrightarrow{{}}\underset{\text{2-Nitroethanol}}{\mathop{C{{H}_{2}}OH.C{{H}_{2}}N{{O}_{2}}}}\,\]
(xxiii) Addition of Acetyl chloride :
\[\underset{\text{Ethene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,+C{{H}_{3}}COCl\xrightarrow{{}}\underset{\text{4-Chlorobutanone-2}}{\mathop{C{{H}_{2}}ClC{{H}_{2}}COC{{H}_{3}}}}\,\]
(4) Uses
(i) For the manufacture of polythene – a plastic material; (ii) For artificial ripening of fruits; (iii) As a general anaesthetic; (iv) As a starting material for a large number of compounds such as glycol, ethyl halides, ethyl alcohol, ethylene oxide, etc; (v) For making poisonous mustard gas (War gas); (vi) For making ethylene-oxygen flame.
You need to login to perform this action.
You will be redirected in
3 sec
