Styrene \[({{C}_{6}}{{H}_{5}}CH=C{{H}_{2}})\text{ }\]
Category : JEE Main & Advanced
It is present in storax balsam and coal-tar in traces.
(1) Preparation
(i) Dehydrogenation of side chain of ethylbenzene :
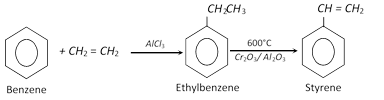
(ii) Decarboxylation of cinnamic acid : This is the laboratory preparation and involves heating of cinnamic acid with a small amount of quinol.
\[{{C}_{6}}{{H}_{5}}CH=CHCOOH\xrightarrow{\text{Quinol}}{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}+C{{O}_{2}}\]
(iii) Dehydration of 1-phenyl ethanol with \[{{H}_{2}}S{{O}_{4}}\] :
\[{{C}_{6}}{{H}_{5}}CHOHC{{H}_{3}}\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}}}}}\,{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\]
(iv) Dehydration of 2-phenyl ethanol with \[ZnC{{l}_{2}}\] :
\[{{C}_{6}}{{H}_{5}}C{{H}_{2}}C{{H}_{2}}OH\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{ZnC{{l}_{2}},\,\text{heat}}}}\,{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\]
(v) Dehydrohalogenation of 1-phenyl-1-chloro ethane : On heating with alcoholic potassium hydroxide, a molecule of hydrogen chloride is eliminated by the chloroderivative.
\[{{C}_{6}}{{H}_{5}}CHClC{{H}_{3}}\underset{\text{Heat}}{\mathop{\xrightarrow{\text{Alc}\text{.}\,KOH}}}\,{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\]
(2) Properties : It is a colourless liquid, boiling point \[{{145}^{o}}C\]. On keeping, it gradually changes into a solid polymer called metastyrene. The polymerisation is rapid in sunlight or when treated with sodium. It shows properties of benzene ring (Electrophilic substitution) and unsaturated side chain (Electrophilic addition). However, the side chain double bond is more susceptible to electrophilic attack as compared to benzene ring.
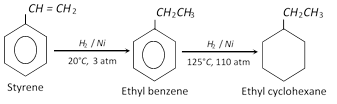
At lower temperature and pressure, it reacts with hydrogen to produce ethylbenzene and at higher temperature and pressure, it is converted into ethyl cyclohexane.
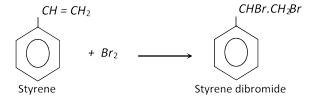
With bromine, it gives the dibromide.
Halogen acids add to the side chain.
\[{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}+HX\xrightarrow{{}}{{C}_{6}}{{H}_{5}}CHXC{{H}_{3}}\]
Preparation of ring substituted styrenes is not done by direct halogenation but through indirect route.
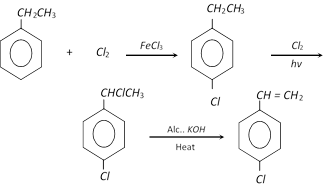
When oxidised under drastic conditions, the side chain is completely oxidised to a carboxyl group.

In presence of peroxides, styrene undergoes free radical polymerisation resulting in the formation of polystyrene ? an industrially important plastic.
\[n{{C}_{6}}{{H}_{5}}CH=C{{H}_{2}}\xrightarrow{\text{Peroxide}}{{\left[ \underset{{{C}_{6}}{{H}_{5\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{-CH-C{{H}_{2}}-}}\,}}\, \right]}_{n}}\]
Co-polymers of styrene with butadiene and other substances are also important since many of them are industrially useful products such as SBR (A rubber substitute).
You need to login to perform this action.
You will be redirected in
3 sec
