Quantum Numbers
Category : JEE Main & Advanced
An atom contains large number of shells and subshells. These are distinguished from one another on the basis of their size, shape and orientation (direction) in space. The parameters are expressed in terms of different numbers called quantum number.
Quantum numbers may be defined as a set of four number with the help of which we can get complete information about all the electrons in an atom. It tells us the address of the electron i.e. location, energy, the type of orbital occupied and orientation of that orbital.
(1) Principal Quantum number (n) : This quantum number determines the main energy level or shell in which the electron is present. The average distance of the electron from the nucleus and the energy of the electron depends on it.
\[{{E}_{n}}\propto \frac{1}{{{n}^{2}}}\] and \[{{r}_{n}}\propto {{n}^{2}}\] (in H-atom)
The principal quantum number takes whole number values, n = 1, 2, 3, 4,... \[\infty \]
(2) Orbital quantum number (l) or azimuthal quantum number \[(l)\] : This represents the number of subshells present in the main shell. These subsidiary orbits within a shell will be denoted as 1, 2, 3, 4 ? or s, p, d, f ... This tells the shape of the subshells.
The orbital angular momentum of the electron is given as \[L=\sqrt{l(l+1)}\,\frac{h}{2\pi }\] (for a particular value of n).
For a given value of n the possible values of \[l\] are \[l\] = 0, 1, 2, ... upto \[(n-1)\]
(3) Magnetic quantum number \[({{m}_{l}})\] : An electron due to it's angular motion around the nucleus generates an electric field. This electric field is expected to produce a magnetic field. Under the influence of external magnetic field, the electrons of a subshell can orient themselves in certain preferred regions of space around the nucleus called orbitals.
The magnetic quantum number determines the number of preferred orientations of the electron present in a subshell.
The angular momentum quantum number m can assume all integral value between \[-l\] to \[+l\] including zero. Thus \[{{m}_{l}}\] can be \[-1,\,\,0,\,\,+\,\,1\] for \[l=1\]. Total values of \[{{m}_{l}}\] associated with a particular value of \[l\] is given by \[(2l+1)\].
(4) Spin (magnetic) quantum number \[({{m}_{s}})\] : An electron in atom not only revolves around the nucleus but also spins about its own axis. Since an electron can spin either in clockwise direction or in anticlockwise direction. Therefore for any particular value of magnetic quantum number, spin quantum number can have two values, i.e. \[{{m}_{s}}=\frac{1}{2}\] (Spin up) or \[{{m}_{s}}=-\frac{1}{2}\] (Spin down)
This quantum number helps to explain the magnetic properties of the substance.
Quantum states of the hydrogen atom
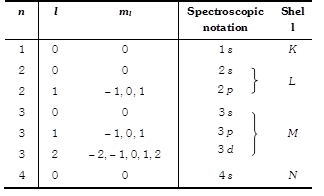
You need to login to perform this action.
You will be redirected in
3 sec
