Cell
Category : JEE Main & Advanced

The device which converts chemical energy into electrical energy is known as electric cell. Cell is a source of constant emf but not constant current.
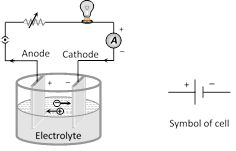
(1) Emf of cell (E) : The potential difference across the terminals of a cell when it is not supplying any current is called it's emf.
(2) Potential difference (V) : The voltage across the terminals of a cell when it is supplying current to external resistance is called potential difference or terminal voltage. Potential difference is equal to the product of current and resistance of that given part i.e. \[V=iR\].
(3) Internal resistance (r) : In case of a cell the opposition of electrolyte to the flow of current through it is called internal resistance of the cell. The internal resistance of a cell depends on the distance between electrodes \[(r\propto d),\] area of electrodes \[[r\propto (1/A)]\] and nature, concentration \[(r\propto C)\] and temperature of electrolyte \[[r\propto (1/temp.)]\].
A cell is said to be ideal, if it has zero internal resistance.
You need to login to perform this action.
You will be redirected in
3 sec
