Mean Free Path
Category : JEE Main & Advanced
(1) The distance travelled by a gas molecule between two successive collisions is known as free path.
\[\lambda =\frac{\text{Total distance travelled by a gas molecule between successive collisions}}{\text{Total number of collisions}}\]
During two successive collisions, a molecule of a gas moves in a straight line with constant velocity and
Let \[{{\lambda }_{1}},{{\lambda }_{2}},{{\lambda }_{3}}.....\] be the distance travelled by a gas molecule during n collisions respectively, then the mean free path of a gas molecule is given by
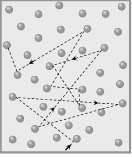
\[\lambda =\frac{{{\lambda }_{1}}+{{\lambda }_{2}}+{{\lambda }_{3}}+....+{{\lambda }_{n}}}{n}\]
(2) \[\lambda =\frac{1}{\sqrt{2}\pi n{{d}^{2}}}\]
where d = Diameter of the molecule,
n = Number of molecules per unit volume
(3) As \[PV=\mu RT=\mu NkT\Rightarrow \frac{N}{V}=\frac{P}{kT}=n=\]Number of molecule per unit volume so \[\lambda =\frac{1}{\sqrt{2}}\frac{kT}{\pi {{d}^{2}}P}\].
(4) From \[\lambda =\frac{1}{\sqrt{2}\pi n{{d}^{2}}}=\frac{m}{\sqrt{2}\pi (mn){{d}^{2}}}\]\[=\frac{m}{\sqrt{2}\pi {{d}^{2}}\rho }\]
[As m = Mass each molecule, mn = Mass per unit volume = Density \[=\rho \]]
(5) If average speed of molecule is v then \[\lambda =v\times \frac{t}{N}\]\[=v\times T\]
[As N = Number of collision in time t, T = time interval between two collisions].
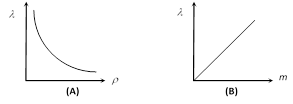
(i) As \[\lambda \propto \frac{1}{\rho }\] and \[\lambda \propto \,m\] i.e. the mean free path is inversely proportional to the density of a gas and directly proportional to the mass of each molecule.
(ii) As \[\lambda =\frac{1}{\sqrt{2}}\frac{kT}{\pi {{d}^{2}}P}\]. For constant volume and hence constant number density n of gas molecules, \[\frac{P}{T}\] is constant so that l will not depend on P and T. But if volume of given mass of a gas is allowed to change with P or T then \[\lambda \propto T\] at constant pressure and \[\lambda \propto \frac{1}{P}\] at constant temperature.

You need to login to perform this action.
You will be redirected in
3 sec
