Real Gases
Category : JEE Main & Advanced
(1) The gases actually found in nature are called real gases.
(2) They do not obeys gas Laws.
(3) For exactly one mole of an ideal gas \[\frac{PV}{RT}=1.\] Plotting the experimentally determined value of \[\frac{PV}{RT}\] for exactly one mole of various real gases as a function of pressure P, shows a deviation from identity.
(4) The quantity \[\frac{PV}{RT}\] is called the compressibility factor and should be unit for an ideal gas.
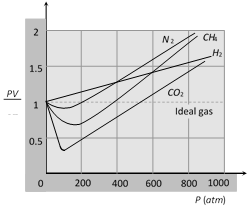
(5) Deviation from ideal behaviour as a function of temperature
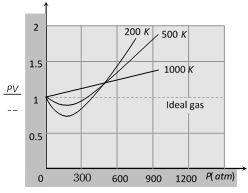
(6) A real gas behaves as ideal gas most closely at low pressure and high temperature. Also can actual gas can be liquefied most easily which deviates most from ideal gas behaviour at low temperature and high pressure.
(7) Equation of state for real gases : It is given by Vander Waal's with two correction in ideal gas equation. The it know as Vander Waal's gas equation.
(i) Volume correction : Due to finite size of molecule, a certain portion of volume of a gas is covered by the molecules themselves. Therefore the space available for the free motion of molecules of gas will be slightly less than the volume V of a gas. Hence the effective volume becomes \[(V-b)\].
(ii) Pressure correction : Due to intermolecular force in real gases, molecule do not exert that force on the wall which they would have exerted in the absence of intermolecular force. Therefore the observed pressure P of the gas will be less than that present in the absence of intermolecular force. Hence the effective pressure becomes \[\left( P+\frac{a}{{{V}^{2}}} \right)\].
(iii) Vander Waal's gas equations
For 1 mole of gas \[\left( P+\frac{a}{{{V}^{2}}} \right)\,(V-b)=RT\]
For \[\mu \] moles of gas \[\left( P+\frac{a{{\mu }^{2}}}{{{V}^{2}}} \right)\ (V-\mu b)=\mu \,RT\]
Here a and b are constant called Vander Waalís constant.
Dimension : \[[a]=[M{{L}^{5}}{{T}^{-2}}]\] and \[[b]=[{{L}^{3}}]\]
Units : \[a=N\times {{m}^{4}}\] and \[b={{m}^{3}}\].
(8) Andrews curves : The pressure (P) versus volume (V) curves for actual gases are called Andrews curves.
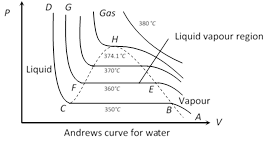
(i) At \[{{350}^{o}}C,\] part AB represents vapour phase of water, in this part Boyle?s law is obeyed \[\left( P\propto \frac{1}{V} \right)\]. Part BC represents the co-existence of vapour and liquid phases. At point C, vapours completely change to liquid phase. Part CD is parallel to pressure axis which shows that compressibility of the water is negligible.
(ii) At \[{{360}^{o}}C\] portion representing the co-existence of liquid vapour phase is shorter.
(iii) At \[{{370}^{o}}C\] this portion is further decreased.
(iv) At \[{{374.1}^{o}}C,\] it reduces to point (H) called critical point and the temperature \[{{374.1}^{o}}C\] is called critical temperature \[({{T}_{c}})\] of water.
(v) The phase of water (at \[{{380}^{o}}C\]) above the critical temperature is called gaseous phase.
(9) Critical temperature, pressure and volume : The point on the P-V curve at which the matter gets converted from gaseous state to liquid state is known as critical point. At this point the difference between the liquid and vapour vanishes i.e. the densities of liquid and vapour become equal.
(i) Critical temperature \[({{T}_{r}})\] : The maximum temperature below which a gas can be liquefied by pressure alone is called critical temperature and is characteristic of the gas. A gas cannot be liquefied if its temperature is more than critical temperature.
\[C{{O}_{2}}\left( 31.1{}^\circ C \right),{{O}_{2}}\left( 118{}^\circ C \right),{{N}_{2}}~\left( 147.1{}^\circ C \right)\] and \[{{H}_{2}}O\left( 374.1{}^\circ C \right)\]
(ii) Critical pressure \[({{P}_{c}})\] : The minimum pressure necessary to liquify a gas at critical temperature is defined as critical pressure \[C{{O}_{2}}(73.87\,\,bar)\] and \[{{O}_{2}}\] (49.7atm)
(iii) Critical volume \[({{V}_{c}})\] : The volume of 1 mole of gas at critical pressure and critical temperature is defined as critical volume \[C{{O}_{2}}(95\times {{10}^{-6}}{{m}^{3}})\]
(iv) Relation between Vander Waal?s constants and \[{{T}_{c}},\,{{P}_{c}},\,{{V}_{c}}\]
\[{{T}_{c}}=\frac{8a}{27Rb}\], \[{{P}_{c}}=\frac{a}{27{{b}^{2}}}\], \[{{V}_{c}}=3b\],
\[a=\frac{27{{R}^{2}}}{64}\frac{T_{c}^{2}}{{{P}_{c}}}\], \[b=\frac{R}{8}\left( \frac{{{T}_{c}}}{{{P}_{c}}} \right)\] and \[\frac{{{P}_{c}}{{V}_{c}}}{{{T}_{c}}}=\frac{3}{8}R\]
You need to login to perform this action.
You will be redirected in
3 sec
