Alkynes
Category : JEE Main & Advanced
These are the acyclic hydrocarbons which contain carbon-carbon triple bond are called alkynes. General formula is \[{{C}_{n}}{{H}_{2n-2}}\]. Ex. Ethyne \[CH\equiv CH\]; Propyne \[C{{H}_{3}}-C\equiv CH\]
(1) General methods of preparation

Preparation of higher alkynes (by metal acetylide)
(2) Physical properties
(i) Acetylene is a colourless gas. It has a garlic odour. The odour is due to presence of impurities of phosphorous and hydrogen sulphide. However, pure acetylene has pleasant odour.
(ii) It is insoluble in water but highly soluble in acetone and alcohol. Acetylene is transported under high pressure in acetone soaked on porous material packed in steel cylinders.
(iii) Its boiling point is \[-{{84}^{o}}C\].
(iv) It is lighter than air. It is somewhat poisonous in nature.
(v) It burns with luminous flame and forms explosive mixture with air.
(3) Chemical reactivity of alkynes : \[C\equiv C\] is less reactive than the carbon-carbon double bond towards electrophilic addition reaction. This is because in alkyne carbon has more S-character so more strongly will be the attraction for \[\pi \] electrons. Alkyne also undergo nucleophilic addition with electron rich reagents. Ex. Addition of water, cyanide, carboxylic acid, alcohols. Nucleophilic addition can be explained on the basis that alkynes form vinylic carbanion which is more stable than alkyl carbanion formed by alkene
\[-C\equiv C-\,+N{{u}^{-}}\xrightarrow{{}}-\overset{Nu}{\mathop{\overset{|\,\,\,\,}{\mathop{C=}}\,}}\,\overset{}{\mathop{C}}\,-\]
Vinylic carbanion (more stable)
\[-C=C-\,\,+N{{u}^{-}}\xrightarrow{{}}\,\,\,-\overset{Nu}{\mathop{\overset{|\,\,\,\,}{\mathop{C-}}\,}}\,\overset{}{\mathop{C}}\,-\]
(alkyl carbanion) (less stable)
(i) Acidity of alkynes : Acetylene and other terminal alkynes (1- alkynes) are weakly acidic in character
Ex. \[CH\equiv CH+NaN{{H}_{2}}\xrightarrow{{}}H-C\equiv \overline{C}\,N{{a}^{+}}+\frac{1}{2}{{H}_{2}}\]
(Monosodium acetylide)
The acetylenic hydrogen of alkynes can be replaced by copper (I) and silver (I) ions. They react with ammonical solutions of cuprous chloride and silver nitrate to form the corresponding copper and silver alkynides.
\[CH\equiv CH+2[Cu{{(N{{H}_{3}})}_{2}}]Cl\xrightarrow{{}}Cu-C\equiv C-Cu+2N{{H}_{4}}Cl+2N{{H}_{3}}\]
Dicopper acetylide (Red ppt)
\[CH\equiv CH+2[Ag{{(N{{H}_{3}})}_{2}}]N{{O}_{3}}\xrightarrow{{}}AgC\equiv C-Ag+2N{{H}_{4}}N{{O}_{3}}+2N{{H}_{3}}\]
Disilver acetylide (white ppt)
This reaction can be used to distinguish between 2-alkynes and 1-alkynes. 1-alkynes will give this test while 2-alkynes, will not give this test.
\[\underset{\text{1-propyne}}{\mathop{C{{H}_{3}}-C\equiv CH}}\,+2[Ag{{(N{{H}_{3}})}_{2}}]\,N{{O}_{3}}\xrightarrow{{}}C{{H}_{3}}-C\equiv C-Ag\]
\[C{{H}_{3}}-C\equiv C-C{{H}_{3}}+2[Ag{{(N{{H}_{3}})}_{2}}]N{{O}_{3}}\xrightarrow{{}}\]No reaction
Explanation for the acidic character : It explained by \[sp\] hybridisation. We know that an electron in \[s-\]orbital is more tightly held than in a \[p\]-orbital. In \[sp\] hybridisation \[s\]-character is more (50%) as compared to \[s{{p}^{2}}\](33%) or \[s{{p}^{3}}\](25%), due to large \[s\]-character the carbon atom is quite electronegative.
(ii) Reaction with formaldehyde
\[\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,OH}{\mathop{\underset{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,|}{\mathop{HC\equiv CH+2C{{H}_{2}}O\xrightarrow{{}}C{{H}_{2}}-C\equiv C}}\,}}\,-\underset{OH}{\mathop{\underset{|\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,}}\,\xrightarrow{Li/N{{H}_{3}}}C{{H}_{2}}-CH=CH-C{{H}_{2}}OH\]
\[\underset{OH\,\,\,\,\,}{\mathop{\underset{|\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}-}}\,}}\,CH=CH-C{{H}_{2}}OH\] [Trans-product ]
(4) Chemical properties of acetylene

Oxidative–Hydroboration : Alkynes react with \[B{{H}_{3}}\] (in THF) and finally converted into carbonyl compounds.
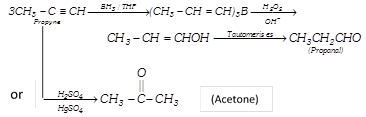
Thus it is useful for preparing aldehyde from terminal alkyne.
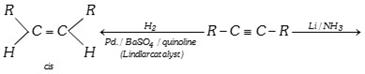
Reduction of Alkyne : Alkynes add on hydrogen in presence of suitable catalysts like finely divided Ni, Pd.
\[CH\equiv CH+{{H}_{2}}\xrightarrow{Ni}C{{H}_{2}}=C{{H}_{2}}\underset{{{H}_{2}}}{\mathop{\xrightarrow{Ni}}}\,C{{H}_{3}}-C{{H}_{3}}\]
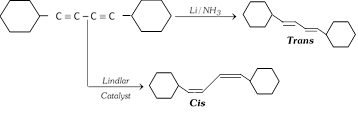
If the triple bond is not present at the end of the carbon chain of the molecule, the alkene formed may be cis and trans depending upon the choice of reducing agents.
With \[Na/N{{H}_{3}}\] or \[Li/N{{H}_{3}}\] in (liquid ammonia) trans alkene is almost an exclusive product while catalytic reduction at alkyne affords mainly cis alkenes.

Degree of unsaturation : The number of degree of unsaturation in a hydrocarbon is given by
\[\frac{2{{n}_{1}}+2-{{n}_{2}}}{2}\], Where \[{{n}_{1}}\] is the number of carbon atoms; \[{{n}_{2}}\] is the number of hydrogen atoms.
For example in \[{{C}_{6}}{{H}_{12}}\], the degree of unsaturation is \[=\frac{2\times 6+2-12}{2}=1\]
Tests of unsaturation
(a) Baeyer’s reagent : It is 1% \[KMn{{O}_{4}}\] solution containing sodium carbonate. It has pink colour. An aqueous solution of the compound, a few drops of Baeyer’s reagent are added, the pink colour of the solution disappears. The decolourisation of pink colour indicates the presence of unsaturation in the compound.
 don't show this test.
don't show this test.
(b) Bromine- carbon tetrachloride test : The compound is dissolved in carbon tetrachloride or chloroform and then a few drops of 5% bromine solution in carbon tetrachloride are added to it, the colour of bromine disappears. It indicates the presence of unsaturation.
(5) Uses
(i) Acetylene is used as an illuminant.
(ii) It is used for the production of oxy-acetylene flame. The temperature of the flame is above \[{{3000}^{o}}C\]. Is employed for cutting and welding of metals.
(iii) Acetylene is used for artificial ripening of fruits.
(iv) It is used as a general anaesthetic under the name naracylene.
(v) Acetylene has synthetic applications. It serves as a starting material for the manufacture of a large variety of substances.
(vi) On electrical decomposition acetylene produces finely divided carbon and hydrogen. Hydrogen is used in airships. \[{{C}_{2}}{{H}_{2}}\xrightarrow{{}}2C+{{H}_{2}}\]
(6) Interconversion
(i) Conversion of ethane into ethene : (Alkane into alkene)
\[\underset{\text{Ethane}}{\mathop{C{{H}_{3}}-C{{H}_{3}}}}\,\underset{hv}{\mathop{\xrightarrow{B{{r}_{2}}}}}\,\underset{\text{Ethyl bromide}}{\mathop{{{C}_{2}}{{H}_{5}}Br}}\,\underset{KOH}{\mathop{\xrightarrow{Alc.}}}\,\underset{\text{Ethene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,\]
(ii) Ethene into ethane : (Alkene into alkane)
\[\underset{\text{Ethene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,\underset{Ni,\,{{300}^{o}}C}{\mathop{\xrightarrow{{{H}_{2}}}}}\,\underset{\text{Ethane}}{\mathop{C{{H}_{3}}-C{{H}_{3}}}}\,\]
(iii) Ethane into ethyne (acetylene) : i.e., alkane into alkyne
\[\underset{\text{Ethane}}{\mathop{C{{H}_{3}}-C{{H}_{3}}}}\,\underset{hv}{\mathop{\xrightarrow{B{{r}_{2}}}}}\,C{{H}_{3}}C{{H}_{2}}Br\underset{KOH}{\mathop{\xrightarrow{Alc.}}}\,\underset{\text{Ethene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,\underset{CC{{l}_{4}}}{\mathop{\xrightarrow{B{{r}_{2}}}}}\,\underset{\text{1, 2-Dibromoethane}}{\mathop{C{{H}_{2}}Br-C{{H}_{2}}Br}}\,\underset{\text{or}\,NaN{{H}_{2}}}{\mathop{\xrightarrow{Alc.KOH}}}\,\underset{\text{Ethyne}}{\mathop{CH\equiv CH}}\,\]
\[\underset{\text{1, 2-Dibromoethane}}{\mathop{C{{H}_{2}}Br-C{{H}_{2}}Br}}\,\underset{\text{or}\,NaN{{H}_{2}}}{\mathop{\xrightarrow{Alc.KOH}}}\,\underset{\text{Ethyne}}{\mathop{CH\equiv CH}}\,\]
(iv) Ethyne into ethane : (Alkyne into alkane)
\[\underset{\text{Ethyne}}{\mathop{CH\equiv CH}}\,\underset{Ni,\,{{300}^{o}}C}{\mathop{\xrightarrow{{{H}_{2}}}}}\,\underset{\text{Ethene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,\underset{Ni,\,{{300}^{o}}C}{\mathop{\xrightarrow{{{H}_{2}}}}}\,\underset{\text{Ethane}}{\mathop{C{{H}_{3}}-C{{H}_{3}}}}\,\]
(v) Ethene into propene : Ascending in alkene series
\[\underset{\text{Ethene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,\xrightarrow{HI}\underset{\text{Iodoethane}}{\mathop{C{{H}_{3}}C{{H}_{2}}I}}\,\xrightarrow{KCN}\underset{\text{(Ethyl cyanide)}}{\mathop{\underset{\text{Propane nitrile}}{\mathop{C{{H}_{3}}C{{H}_{2}}CN}}\,}}\,\underset{\text{Reduction}}{\mathop{\xrightarrow{[H]}}}\,\]
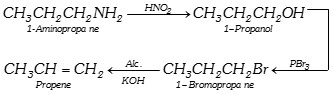
or
\[\underset{\text{Ethene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,\xrightarrow{HI}\underset{\text{Iodoethane}}{\mathop{C{{H}_{3}}C{{H}_{2}}I}}\,\xrightarrow{Li{{(C{{H}_{3}})}_{2}}Cu}\underset{\text{Propane}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{3}}}}\,\]
\[\underset{hv}{\mathop{\xrightarrow{C{{l}_{2}}}}}\,\underset{\text{1-Chloro propane}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Cl}}\,\underset{KOH}{\mathop{\xrightarrow{Alc.}}}\,\underset{\text{Propene}}{\mathop{C{{H}_{3}}CH=C{{H}_{2}}}}\,\]
or
\[C{{H}_{2}}=C{{H}_{2}}\xrightarrow{HI}C{{H}_{3}}C{{H}_{2}}I\xrightarrow{C{{H}_{3}}I/Na}\underset{\text{Propane}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{3}}}}\,\]
\[\underset{hv}{\mathop{\xrightarrow{C{{l}_{2}}}}}\,\underset{\text{1-Chloro propane}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}Cl}}\,\underset{KOH}{\mathop{\xrightarrow{Alc.}}}\,\underset{\text{Propene}}{\mathop{C{{H}_{3}}CH=C{{H}_{2}}}}\,\]
(vi) Propene into ethene : Descending an alkene series
\[\underset{\text{Propene}}{\mathop{C{{H}_{3}}-CH=C{{H}_{2}}}}\,\xrightarrow{{{O}_{3}}/{{H}_{2}}O}\underset{\text{Ethanal}}{\mathop{C{{H}_{3}}CHO}}\,\underset{LiAl{{H}_{4}}}{\mathop{\xrightarrow{[H]}}}\,\underset{\text{Ethanal}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,\underset{{{170}^{o}}C}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}}}}}\,\underset{\text{Ethene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,\]
\[\underset{\text{Ethanol}}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,\underset{{{170}^{o}}C}{\mathop{\xrightarrow{{{H}_{2}}S{{O}_{4}}}}}\,\underset{\text{Ethene}}{\mathop{C{{H}_{2}}=C{{H}_{2}}}}\,\]
(vii) Acetylene into propyne (methyl acetylene) : (Ascent)
\[\underset{\text{Acetylene}}{\mathop{CH\equiv CH}}\,\xrightarrow{Na}\underset{\text{acetylide}}{\mathop{\underset{\text{Monosodium }}{\mathop{CH\equiv CNa}}\,}}\,\xrightarrow{C{{H}_{3}}I}\underset{\text{Propyne}}{\mathop{CH\equiv C-C{{H}_{3}}}}\,\]
(viii) Propyne into acetylene : (Descent)
\[\underset{\text{Propyne}}{\mathop{C{{H}_{3}}C=CH}}\,\xrightarrow{\text{Lindlar }\!\!'\!\!\text{ s catalyst}}\underset{\text{Propylene}}{\mathop{C{{H}_{3}}CH=C{{H}_{2}}}}\,\xrightarrow{{{O}_{3}}/{{H}_{2}}O}\]
\[\underset{\text{Acetaldehyde}}{\mathop{C{{H}_{3}}CHO}}\,\xrightarrow{PC{{l}_{5}}}\underset{\text{chloride}}{\mathop{\underset{\text{Ethylidene}}{\mathop{C{{H}_{3}}CHC{{l}_{2}}}}\,}}\,\underset{KOH}{\mathop{\xrightarrow{Alc.}}}\,\underset{\text{Acetylene}}{\mathop{CH\equiv CH}}\,\]
(ix) 1-Butyne into 2-pentyne : (Ascent)
\[\underset{1-\text{Butyne}}{\mathop{C{{H}_{3}}C{{H}_{2}}C\equiv CH}}\,\xrightarrow{NaN{{H}_{2}}}C{{H}_{3}}C{{H}_{2}}C\equiv C-Na\xrightarrow{C{{H}_{3}}I}\]
\[\underset{2-\text{Pentyne}}{\mathop{C{{H}_{3}}C{{H}_{2}}-C\equiv CC{{H}_{3}}}}\,\]
(x) 1-Butyne into 2-pentanone : (Not more than three steps)
\[\underset{\text{1-Butyne}}{\mathop{C{{H}_{3}}C{{H}_{2}}C\equiv CH}}\,\underset{(Liq.\,N{{H}_{3}})}{\mathop{\xrightarrow{NaN{{H}_{2}}}}}\,C{{H}_{3}}C{{H}_{2}}C\equiv CNa\xrightarrow{C{{H}_{3}}I}\]
\[\underset{2-\text{Pentyne}}{\mathop{C{{H}_{3}}C{{H}_{2}}C\equiv CC{{H}_{3}}}}\,\underset{HgS{{O}_{4}}}{\mathop{\xrightarrow{{{H}_{2}}O,\,{{H}_{2}}S{{O}_{4}}}}}\,\underset{2-\text{Pentanone}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,C{{H}_{3}}}}\,\]
You need to login to perform this action.
You will be redirected in
3 sec
