Bi-phenyl \[{{C}_{6}}{{H}_{5}}\text{ }{{C}_{6}}{{H}_{5}}\]
Category : JEE Main & Advanced
It occurs in coal-tar. It is the simplest example of an aromatic hydrocarbon in which two benzene rings are directly linked to each other.
(1) Methods of formation
(i) Fittig reaction : It consists heating of an ethereal solution of bromobenzene with metallic sodium.
![]()
(ii) Ullmann biaryl synthesis : Iodobenzene, on heating with copper in a sealed tube, forms biphenyl. The reaction is facilitated if a strong electron withdrawing group is present in ortho or para position.
![]()
(iii) Grignard reaction : Phenyl magnesium bromide reacts with bromo benzene in presence of \[CoC{{l}_{2}}\].
![]()
![]()
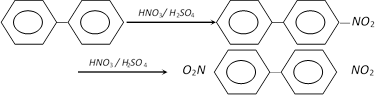
(2) Properties : It is a colourless solid, melting point 71°C. It undergoes usual electrophilic substitution reactions. Since aryl groups are electron withdrawing , they should have deactivating and m-orientating effect. But, it has been experimentally shown that presence of one benzene ring activates the other for electrophilic substitution and directs the incoming group to o- and p- positions. It has been shown that monosubstitution in the bi-phenyl results in the formation of para isomer as the major product.
Another special feature of the biphenyl is the behaviour towards second substitution in a monosubstituted biphenyl. The second substituent invariably enters the unsubstituted ring in the ortho and para position no matter what is the nature of substituent already present.
You need to login to perform this action.
You will be redirected in
3 sec
