Anaerobic Respiration
Category : NEET
Anaerobic respiration first studied by Kostychev (1902), Anaerobic respiration is an enzyme-controlled, partial break down of organic compounds (food) without using oxygen and releasing only a fraction of the energy. It is also called intra-molecular respiration (Pfluger, 1875). Anaerobic respiration occurs in the roots of some water-logged plants, certain parasitic worms (Ascaris and Taenia), animal muscle and some microorganisms (bacteria, moulds). In microorganisms anaerobic respiration is often called fermentation.
Higher organism like plants cannot perform anaerobic respiration for long. It is toxic because accumulation of end products, insufficient amount of available energy and causes stoppage of many active process.
(1) Process of anaerobic respiration : In this process pyruvate which is formed by glycolysis is metabolised into ethyl alcohol or lactic acid and \[C{{O}_{2}}\]in the absence of oxygen. Glycolysis is occurs in cytoplasm so the site of anaerobic respiration is cytoplasm.
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}2{{C}_{2}}{{H}_{5}}OH+\text{ }2C{{O}_{2}}+\text{ }52\text{ }Kcal/218.4kJ\]
(i) Formation of ethyl alcohol : When oxygen is not available, yeast and some other microbes convert pyruvic acid into ethyl alcohol. This is two step
process as explained below

(a) In the first step pyruvic acid is decarboxylated to yield acetaldehyde and \[C{{O}_{2}}\]. In the presence of Mg++ and TPP (Thiamine pyrophosphate) pyruvate carboxylase.
\[\underset{(\text{Pyruvic}\,\text{acid})}{\mathop{C{{H}_{3}}COCOOH}}\,\underset{\begin{smallmatrix}
\text{Carboxylase} \\
M{{g}^{2+}}+TPP
\end{smallmatrix}}{\mathop{\xrightarrow{\text{Pyruvic}}}}\,\underset{(\text{Acetaldehyde})}{\mathop{C{{H}_{3}}CHO}}\,+C{{O}_{2}}\]
(b) In the second step acetaldehyde is reduced to ethyl alcohol by NADH2 formed in the glycolysis.
\[\underset{(\text{Acetaldehyde})}{\mathop{C{{H}_{3}}CHO}}\,+NAD{{H}_{2}}\underset{\text{dehydrogenase}}{\mathop{\xrightarrow{\text{Alcoholic}}}}\,\underset{(\text{Ethyl}\,\text{alcohol})}{\mathop{{{C}_{2}}{{H}_{5}}OH}}\,+NAD\]
(ii) Production of lactic acid : In this process hydrogen atoms removed from the glucose molecule during glycolysis are added to pyruvic acid molecule and thus lactic acid is formed.
\[\underset{\text{(Pyruvic acid)}}{\mathop{C{{H}_{3}}COCOOH}}\,\to \underset{\text{(Lactic acid)}}{\mathop{C{{H}_{3}}CHOH.COOH}}\,+NAD\]
Lactic acid is produced in the muscle cells of human beings and other animals. During strenuous physical activity such as running, the amount of oxygen delivered to the muscle cells may be insufficient to keep pace with that of cellular respiration. Under such circumstances lactic acid is formed which accumulates in the muscle cells and causes muscle fatigue.
(2) Pasteur effect : Two types of respiration -anaerobic and aerobic respiration produce carbon dioxide in the ratio of 1:3 as shown in the equation.
Anaerobic Respiration : \[\underset{(\text{Two}\,\text{molecules}\,\text{of}\,\,C{{O}_{2}}\,\text{are}\,\text{produced})}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}\to 2{{C}_{2}}{{H}_{5}}OH+2C{{O}_{2}}}}\,\]
Aerobic Respiration : \[\underset{(\text{Six}\,\text{molecules}\,\text{of}\,C{{O}_{2}}\,\text{are}\,\text{produced})}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{4}}+6{{O}_{2}}\to 6{{H}_{2}}O+6C{{O}_{2}}}}\,\]
Pasteur noted that when oxygen is given to the running anaerobic respiration the output of CO2 is not similar to aerobic respiration, i.e. during aerobic respiration the ratio 1:3 does not always appear to be true. In several cases the amount of carbon dioxide is much less in comparison to normal aerobic respiration as shown above. For such cases it is considered that the presence of oxygen may sometimes lower down the rate of breakdown of sugar. The phenomenon is named as 'Pasteur's effects' after the name of great scientist and the process may be defined as "the inhibition of sugar breakdown due to the presence of oxygen under aerobic condition" and the reaction is called Pasteur reaction. Dixon (1937) stated that the Pasteur effect is the action of oxygen is checking the high rate of loss of carbohydrate and in suppressing or diminishing the accumulation of products of fermentation."
Pasteur's effect is said to occur due to many reasons. Some of them are :
(i) Pasteur reaction inhibits some glycolytic enzyme and stops glycolysis.
(ii) Formation of excess of CO2 from degradation of compounds other than respiratory substrate.
(iii) Increased glycolysis with decreased oxygen tension.
(iv) Occurrence of partial oxidative glycolytic products and oxidative anabolism (resynthesis, a process corresponding HMP pathway).
(3) Connection between aerobic and anaerobic respiration
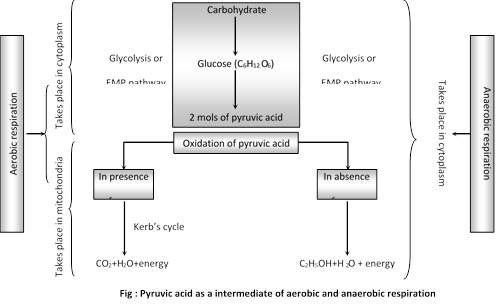
The glycolysis is the common phase and its products pyruvic acid is the common intermediate of the aerobic and anaerobic respiration.
(4) Fermentation : Fermentation is a kind of anaerobic respiration carried out by microorganisms fungi and bacteria. In microorganism the term anaerobic respiration is replaced by fermentation (Cruickshank, 1897) ; which is known after the name of its major product, e.g., alcohol fermentation, lactic acid fermentation.
Gay Lussac was the first to provide following reaction for the fermentation of sugar.
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}=2C{{O}_{2}}+2C{{H}_{3}}C{{H}_{2}}OH+52Kcal\]
Louis Pasteur (1822-1895) supported Gay Lussacs reaction and concluded that fermentation occurred and concluded that fermentation occurred only when living Yeast cells were present.
Buchner (1897) found that yeast extract could perform fermentation of sugary solution. The enzyme complex present in yeast which could perform fermentation was named as zymase. Because of the latter, fermentation is also called zymosis. Besides zymase yeast cells also contain enzymes like sucrose and maltose which can ferment sucrose and maltose respectively. Direct fermentation of starch by yeast is not possible as it lacks amylase enzyme.
The fermentation is of two types : Homofermentive (one product) and heterofermentive (two or more than two types of products). Alcoholic fermentation may occur in almost, any moist sugar containing medium or sugar solution, such as fruit juice, which is inoculated with yeast or which is left exposed to air. The examples of fermentation:
(i) Butyric acid fermentation : It occurs in butter which has turned rancid. Bacteria like Clostridium butyricus and Bacillus butyricus are responsible for fermenting sugars and lactic acid into butyric acid to the following equation :
\[\underset{\text{(hexose)}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,\to \underset{\text{(butyric}\,\text{acid)}}{\mathop{{{C}_{4}}{{H}_{8}}{{O}_{2}}}}\,+2{{H}_{2}}+2C{{O}_{2}}\]
\[\underset{\text{(lactic}\,\text{acid)}}{\mathop{2{{C}_{3}}{{H}_{6}}{{O}_{3}}}}\,\to \underset{\text{(butyric}\,\text{acid)}}{\mathop{{{C}_{4}}{{H}_{8}}{{O}_{2}}}}\,+2{{H}_{2}}+2C{{O}_{2}}\]
(ii) Lactic acid fermentation : In this process the lactose sugar, present in the milk, is converted into lactic acid which provides a distinctive sour taste to the milk. Two bacteria viz., Bacterium lactic acidi and B.acidi lactici take part in this process.
\[\underset{\text{(lactose)}}{\mathop{{{C}_{12}}{{H}_{22}}{{O}_{11}}}}\,+{{H}_{2}}O\to \underset{\text{(glucose)}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,+\underset{\text{(galactose)}}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,\]
\[\underset{{}}{\mathop{\underset{(hexose)}{\mathop{{{C}_{6}}{{H}_{12}}{{O}_{6}}}}\,\to \underset{(lactic\,acid)}{\mathop{2{{C}_{3}}{{H}_{6}}{{O}_{3}}}}\,}}\,\]
(iii) Acetic acid fermentation : It is different from other types of fermentation as it utilises atmospheric oxygen. Acetic acid fermentation is catalysed by Acetabacter aceti, and A. xylinum which oxidised ethyl alcohol into acetic acid.
\[\underset{(\text{ethyl}\,\text{alcohol})}{\mathop{{{C}_{2}}{{H}_{5}}OH}}\,+{{O}_{2}}\to \underset{(\text{acetic}\,\text{acid})}{\mathop{C{{H}_{3}}COOH}}\,+{{H}_{2}}O+\text{energy}\]
(iv) Importance of fermentation : Anaerobic respiration is advantageous in many ways :
(a) It supplement, the energy provided by aerobic respiration during intense muscular activity.
(b) Brewing industry produces beers and wines by fermentation of sugary solution with yeast (saccharomyces cerevisiae).
(c) Baking industry uses CO2 released by Yeast cells in alcoholic fermentation in raising the dough and making bread spongy.
(d) Dairy industry produces yogurt, cheese and butter by fermenting milk sugar lactose to lactic acid with lactic acid bacteria (Streptococcus lactis). Lactic acid coagulates the milk protein casein and fuses droplets of milk fat.
(e) Tea and tobacco leaves are cured (freed of bitterness and imparted pleasant flavours) by fermentation with certain bacteria.
(f) Vinegar is produced by fermenting molasses with yeast to ethyl alcohol which is then oxidised to acetic acid with aerobic acetic acid bacteria (Acetobacter aceti).
(g) Bacterial fermentation is also used for tanning hides (removal of fat, hair and other tissues).
(h) Retting of hemp fibers is achieved by fermentation with Pseudomonas fluroscense.
(i) Ensilase, a nutritive fodder for cattle, is prepared by fermentation with bacteria in air-tight-chambers called silos.
(5) Efficiency of respiration
(i) Efficiency of aerobic respiration : We used the generally accepted amount of 12,000-14,000 calories per mole of ATP approximately 456,000-532,000 calories are generated from one mole of glucose. One mole of glucose contains about 686,000 calories(686 Kcal) of energy in the form of bonds. When one molecule of glucose is oxidised to carbon dioxide and water 673,000 calories of energy released.
However the actual amount of energy available from each ATP (rest of energy is lost as heat, and so on) is approximately 7,3000 calories (7.28 Kcal) or-34 kJ.
Therefore, actual energy yield from one mole of glucose is :
\[=\text{ }38\times 7.3=277.4Kcal\]
So the percent of aerobic respiration = \[\frac{277.4}{686}\times 100\]
= 40.43%
Thus, efficiency of aerobic respiration = 40% approx.
Out of 686 Kcal. of one mole glucose, only 277.4 Kcal. is trapped in the form of ATP.
(ii) Efficiency of anaerobic respiration : In anaerobic respiration of carbohydrate by glycolysis apparently 2ATP molecules are formed per glucose molecule. Therefore, efficiency of anaerobic respiration will be :
\[{{C}_{6}}{{H}_{12}}{{O}_{6}}\to \,2{{C}_{2}}{{H}_{5}}OH+2C{{O}_{2}}+52\,Kcal.\]
Percent of anaerobic respiration = \[\frac{2\times 7.3}{52}\times 100=28.07%\]
(iii) Efficiency of alcoholic fermentation : By Yeast only two molecules of ATP are generated per glucose molecule and efficiency will be, therefore,
\[\]\[{{\text{C}}_{\text{6}}}{{H}_{12}}{{O}_{6}}\to \,2{{C}_{2}}{{H}_{5}}OH+2C{{O}_{2}}+56Kcal.\]
Percent of fermentation = \[=\frac{2\times 7.3}{56}\times 100\text{ }=26.07%\]
(6) Difference between respiration and fermentation
|
Respiration |
Fermentation |
|
It may occur both in the presence and absence of oxygen. |
It does not require oxygen. |
|
Occurs only in living cells. |
It does not occur with in the living cells. It requires only enzymes and substrate. |
|
Sugar is oxidised and CO2 and H2O are formed as end products. |
Different substances oxidise to form alcohol or organic acids. |
|
Complete oxidation of substrate occurs, hence produces large amount of energy. |
Incomplete oxidation of substrate occurs and hence less energy is produced. |
|
It can occur in any living cell. |
It occurs mainly with the help of yeast or bacterial cells. |
Many microbiologists have distinguished Aerobic respiration, Anaerobic respiration and Fermentation from one another principally by the means through which hydrogen is removed from various substrates (the hydrogen donor) and by nature of ultimate substrate accepting this hydrogen (the hydrogen acceptor).
|
Aerobic Respiration |
Anaerobic Respiration |
Fermentation |
|
Molecular oxygen is the ultimate electron acceptor for biological oxidation. The ETS serves to transfer electrons from oxidisable donor to molecular oxygen. The early enzymatic steps involve dehydrogenation whereas the final steps are mediated by a group of enzyme called cytochromes. Ultimately the electrons are transferred to oxygen which is reduced to water. During aerobic respiration ATP is generated by coupled reaction |
The ultimate electron acceptor is an inorganic compound other than oxygen. The compounds accepting the hydrogen (electrons) are nitrates, sulphates, carbonates or CO2. Anaerobic respiration produces ATP through phosphorylation reaction involving electron transfer systems. (mechanism not known) |
The final electron acceptors are organic compounds. Both electron donors (oxidizable substrate) and electron acceptors (oxidizing agent) are organic compounds and usually both substrates arise from same organic molecules during metabolism. Thus part of the nutrient molecule is oxidised and part reduced and the metabolism results in intramolecular electron rearrangement. ATP is generated by substrate level phosphorylation. This reaction differs from oxidative phosphorylation because oxygen itself is not required for ATP generation. |
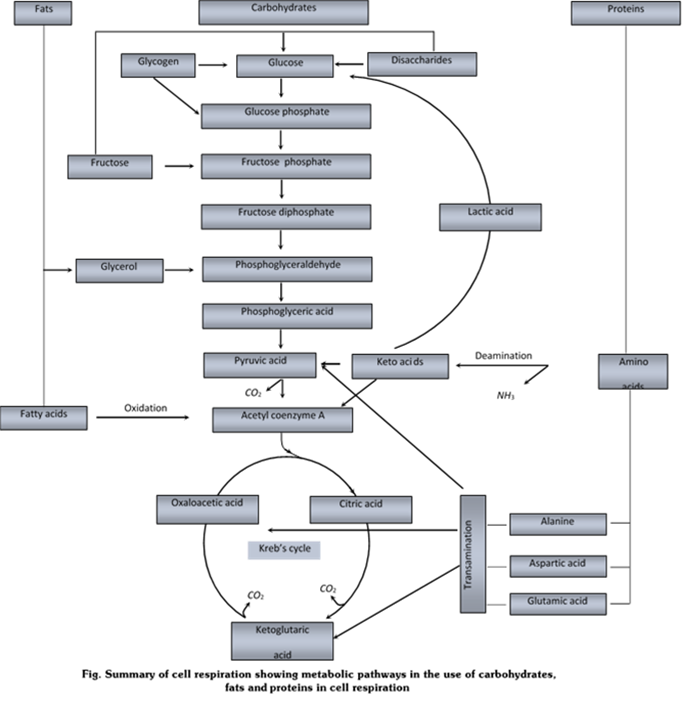
Fat, Protein and Salt respiration
(1) Fat respiration : Fats are stored as triglycerides in cells, (in animal-adipose tissues and in plants-seeds). They break up into fatty acids and glycerol in the cytoplasm before use in respiration. Glycerol converted into Dihydroxy acetonephosphate and enters into glycolysis. The conversion of fatty acid into carbohydrate is called \[\beta \]oxidation. It convert in acetate units of acetyl CoA to glyoxylate and malate (malic acid) takes place in microbodies, termed glyoxysomes. The glyoxysomes are contain all the necessary enzymes for \[\beta \]oxidation of fatty acid to acetyl CoA and subsequent conversion of the acetate units to malic acid (malate) and succinic acid (succinate), the cycle is known as Glyoxylate cycle.
(i) Energy output : A molecule of 18-carbon stearic acid on complete oxidation produces 147 high-energy phosphates. A 6-carbon glucose molecule yields 36 or 38 ATP. With this rate, an 18-carbon molecule is expected to give 3 times more energy (36 or 38×3=108 or 114 ATP) but it provides about 4 times more energy \[\left( 36\text{ }or\text{ }38\text{ }\times 4=144\text{ }or\text{ }152\text{ }ATP \right)\] than 6-carbon glucose produces.
(ii) Glyoxylate cycle
(a) Discovery : Kornberg and Krebs discovered first this cycle in the bacterium Pseudomonas. Later, the reaction of \[\beta \]-oxidation of fatty acids and its conversion of acetyl CoA to glyoxylate and malate occurs in glyoxysomes given by Beevers.
(b) Occurrence : It occurs in seed rich in fats convert stored fats to carbohydrates during germination. The cycle does not appears to be present in those seeds that store starch rather than fat. Glyoxylate activity in germination seeds ceases as soon as the fat reserves have been used up. The fact that plants convert fatty acid to carbohydrates is due to operation of two unique glyoxysome enzyme not known to be present in animals : isocitrate lyase and malate synthetase.
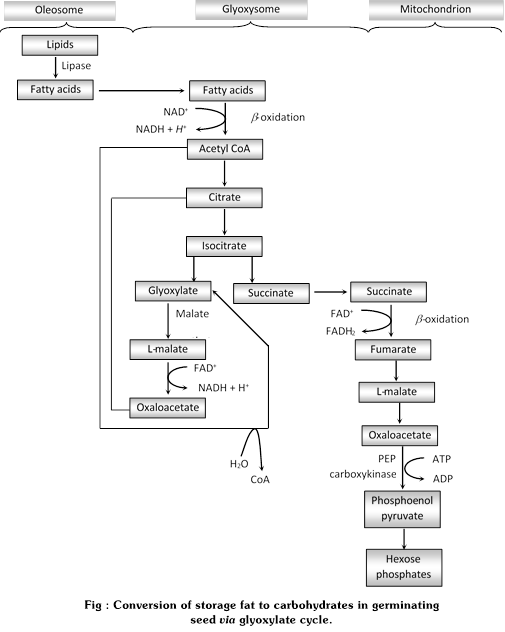
(c) Description : The cycle starts with fatty acids are derived from lipase-mediated enzymolysis of triglycerides occurring in lipid bodies called oleosomes. The fatty acids undergo\[\beta \]oxidation in the glyoxysome with the formation of acetyl CoA.
The acetyl CoA reacts with oxaloacetate to form citrate and then isocitrate. The isocitrate is cleaved to succinate and glycoxylate. This reaction is catalyzed by isocitrate lyase. The glyoxylate combines with acetyl CoA to form malate, the reaction being catalyzed by malate synthetase. The malate in the glyoxysome is oxidized to oxaloacetate, which initiates the cycle again by combining with acetyl CoA derived from \[\beta \]oxidation of fatty acids. The succinate produced moves out of the glyoxysome and into the mitochondrion, where it is converted through the conventional Krebs cycle reactions to oxaloacetate.
The increase of oxaloacetate (OAA) provides ample substrate for amino acid production and carbohydrate formation by reverse glycolysis. Conversion of OAA to phosphoenolpyruvic acid and other glycolytic intermediates takes place in the cytoplasm.
(2) Protein respiration : The proteins split into amino acids in the cytoplasm for use in respiration. The amino acids enter respiratory routes in two ways : Deamination and Transamination.
(i) Deamination : In deamination, an amino acid loses its amino group (-NH2) and changes into a keto acid. The latter may further change into a pyruvic acid or acetyl coenzyme A. Pyruvic acid is oxidised to acetyl coenzyme A. The latter enters the Krebs cycle.
(ii) Transamination : In transamination, an amino group of an amino acid is transferred to an appropriate keto acid, forming a new amino acid and a new keto acid. The keto acids so formed are normal participants of glycolysis or Krebs cycle. Of the all amino acids of plant cell only glutamic acid is believed to be oxidised directly by the enzyme, glutamic acid dehydrogenase into a-ketoglutaric acid and ammonia in the presence of NAD. a-ketoglutaric acid enters the Kreb's cycle to undergo cyclic degration and oxidation.
(3) Salt respiration : This respiration is discovered by Lundegarth and Burstram monovalent chlorides of Na, K and divalant chlorides of Li, Ca and Mg are responsible for salt respiration. According to Lundegarth amount of anion absorbed by plant cells rather than to the absorption of cations of salts, so it is also called anion respiration.
When a fresh water plant transferred to the salty water the rate of respiration increase due to salt respiration. The cause of increase in the rate of respiration during absorption of minerals by roots is also salt respiration.
You need to login to perform this action.
You will be redirected in
3 sec
