Lipids
Category : JEE Main & Advanced
Lipids are constituents of plants and tissues which are insoluble in water but soluble in organic solvents such as chloroform, carbon tetrachloride, ether or benzene. They include a large variety of compounds of varying structures such as oils and fats; phospholipids, steroids, etc. Lipids are mainly made of carbon, hydrogen and oxygen. The number of oxygen atoms in a lipid molecule is always small as compared to the number of carbon atoms. Sometimes small amounts of phosphorus, nitrogen and sulphur are also present. They have a major portion of their structure like a hydrocarbon (aliphatic or fused carbon rings). Lipids serve as energy reserve for use in metabolism and as a major structural material in cell membranes for regulating the activities of cell and tissues.
Simple lipids are esters of glycerol with long chain monocarboxylic acids which can be saturated or unsaturated. These are generally called glycerides of fats and oils. Waxes are esters of fatty acids with certain alcohols, not glycerol. Fats and oils have biological importance but waxes have no value as these are not digested.
The functions of triglycerides are the following
(1) They are energy reserves in the cells and tissues of living system. When digested, triglycerides are hydrolysed to fatty acids and glycerol.
(2) Catabolism of fatty acids form acetyl-coenzyme-A. Most of the energy of fatty acids is converted into ATP.
(3) Acetyl coenzyme is the starting material for the synthesis of many compounds.
(4) Fats deposited beneath the skin and around the internal organs minimize loss of body heat and also act as cushions to absorb mechanical impacts.
Another very important class of lipids are the phospholipids. These are polar lipids and like the fats, are esters of glycerol. In this case, however, only two fatty acid molecules are esterified to glycerol, at the first and second carbon atom. The remaining end position of the glycerol is esterified to a molecule of phosphoric acid, which in turn is also esterified to another alcohol. This gives a general structure.
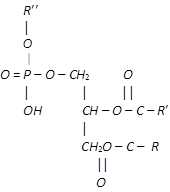
The alcoholic compound linked to phosphoric group may be choline, ethanol, amine, serine or inositol. The phosphate groups forms a polar end, i.e., hydrophilic (water-attracting) and the two fatty acid chains constitute the non-polar tail, i.e., hydrophobic (water repelling). This structure gives the phospholipids good emulsifying and membrane forming properties.
Cell membranes are composed of phopholipids and proteins in about equal, proportion. The phospholipids in the membrane appear to be arranged in a double layer or bilayer in which the non-polar tails face each other, thereby exposing the polar heads to the aqueous environment on either side of the membrane. Proteins found in the membrane are embedded in the mossaic formed by the lipids. Phospholipids facilitate the transport of ions and molecules in and out of the cell and regulate the concentration of molecules and ions within the cell. They provide structural support for certain proteins.
The above mentioned lipids are mainly straight chain compounds. There is a third class of lipids which are not straight chain compounds. They are called Sterols. The sterols are composed of fused hydrocarbon rings and a long hydrocarbon side chain. Cholestrol is most important compound of this class and is found in animals only. It exists either free or as ester with a fatty acid. Cholestrol is also the precursor of hormones. Cholestrol and its esters are insoluble in water. So they are deposited in the arteries and veins if the blood cholestrol rises. This leads to high blood pressure and heart diseases. Cholestrol is a part of animal cell membrane and is used to synthesized steroid hormones, vitamin-D and bile salts.
You need to login to perform this action.
You will be redirected in
3 sec
