Kp and Kc Relationship and Characteristics of K
Category : NEET
Kp & Kc Relationship and Characteristics of K
\[{{K}_{p}}\And {{K}_{c}}\] Relationship
Unit of equilibrium constant : Equilibrium constant K has no units i.e., dimensionless if the total number of moles of the products is exactly equal to the total number of moles of reactants. On the other hand if the number of mioles of products and reactants are not equal, K has specific units.
Units of \[{{K}_{p}}\] and \[{{K}_{c}}\] and the value of \[\Delta n\]
|
Value of Dn |
Relation between \[{{K}_{p}}\] and \[{{K}_{c}}\] |
Units of \[{{K}_{p}}\] |
Units of \[{{K}_{c}}\] |
|
0 |
\[{{K}_{p}}={{K}_{c}}\] |
No unit |
No unit |
|
>0 |
\[{{K}_{p}}>{{K}_{c}}\] |
\[{{\left( atm \right)}^{\Delta n}}\] |
\[{{\left( mole\text{ }{{l}^{1}} \right)}^{\Delta n}}\] |
|
<0 |
\[{{K}_{p}}<{{K}_{c}}\] |
\[{{\left( atm \right)}^{\Delta n}}\] |
\[{{\left( mole\text{ }{{l}^{1}} \right)}^{\Delta n}}\] |
(5) Applications of equilibrium constant: Knowing the value of the equilibrium constant for a chemical reaction is important in many ways. For example, it judge the extent of the reaction and predict the direction of the reaction.
(i) Judging the extent of reaction
We can make the following generalisations concerning the composition of equilibrium mixture.
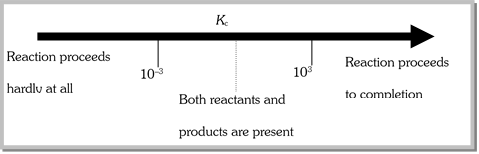
(a) If \[{{K}_{c}}>{{10}^{3}}\], products predominate over reactants. If \[{{K}_{c}}\] is very large, the reaction proceeds almost all the way to completion.
(b) If \[{{K}_{c}}<{{10}^{-3}}\], reactants predominate over products. If \[{{K}_{c}}\] is very small, the reaction proceeds hardly at all.
(c) If \[{{K}_{c}}\] is in the range \[{{10}^{-3}}\] to \[{{10}^{3}}\], appreaciable concentration of both reactants and products are present. This is illustrated as follows,
(ii) Reaction quotient and predicting the direction of reaction : The concentration ratio, i.e., ratio of the product of concentrations of products to that of reactants is also known as concentration quotient and is denoted by Q.
Concentration quotient, \[Q=\frac{[X][Y]}{[A][B]}\].
It may be noted that Q becomes equal to equilibrium constant (K) when the reaction is at the equilibrium state. At equilibrium, \[Q=K={{K}_{c}}={{K}_{p}}\]. Thus,
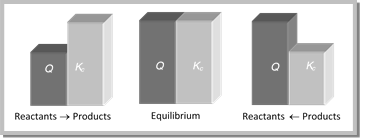
(a) If Q > K, the reaction will proceed in the direction of reactants (reverse reaction).
(b) If Q < K, the reaction will proceed in the direction of the products (forward reaction).
(c) If Q = K, the reaction mixture is already at equilibrium.
Thus, a reaction has a tendency to form products if Q < K and to form reactants if Q > K.
This has also been shown in figure,
(6) Calculation of equilibrium constant : We have studied in the characteristics of the equilibrium constant that its value does not depend upon the original concentrations of the reactants and products involved in the reaction. However, its value depends upon their concentrations at the equilibrium point. Thus, if the equilibrium concentrations of the species taking part in the reaction be known, then the value of the equilibrium constant and vice versa can be calculated.
Homogeneous equilibria and equations for equilibrium constant (Equilibrium pressure is P atm in a V L flask)
|
|
\[\Delta n=0\,;\,\,{{K}_{p}}={{K}_{c}}\] |
\[\Delta n<0\] ; \[{{K}_{p}}<{{K}_{c}}\] |
\[\Delta n>0;\ {{K}_{p}}>{{K}_{c}}\] |
|
|
|
\[\underset{(g)}{\mathop{{{H}_{2}}}}\,\]+ \[\underset{(g)}{\mathop{{{I}_{2}}}}\,\] ? \[\underset{(g)}{\mathop{2HI}}\,\] |
\[\underset{(g)}{\mathop{{{N}_{2}}}}\,+\underset{(g)}{\mathop{3{{H}_{2}}}}\,\]? \[\underset{(g)}{\mathop{2N{{H}_{3}}}}\,\] |
\[\underset{(g)}{\mathop{2S{{O}_{2}}}}\,+\underset{(g)}{\mathop{{{O}_{2}}}}\,\]?\[2\underset{(g)}{\mathop{S{{O}_{3}}}}\,\] |
\[\underset{(g)}{\mathop{PC{{l}_{_{5}}}}}\,\]?\[\underset{(g)}{\mathop{PC{{l}_{3}}}}\,+\underset{(g)}{\mathop{C{{l}_{2}}}}\,\] |
|
Initial mole |
1 1 0 |
1 3 0 |
2 1 0 |
1 0 0 |
|
Mole at Equilibrium |
(1?x) (1? x) 2x |
(1?x) (3?3x) 2x |
(2?2x) (1?x) 2x |
(1?x) x x |
|
Total mole at equilibrium |
2 |
(4 ? 2x) |
(3 ? x) |
(1 + x) |
|
Active masses |
\[\left( \frac{1-x}{V} \right)\] \[\left( \frac{1-x}{V} \right)\] \[\frac{2x}{V}\] |
\[\left( \frac{1-x}{V} \right)\] \[3\,\left( \frac{1-x}{V} \right)\] \[\left( \frac{2x}{V} \right)\] |
\[\left( \frac{2-2x}{V} \right)\] \[\left( \frac{1-x}{V} \right)\] \[\left( \frac{2x}{V} \right)\] |
\[\left( \frac{1-x}{V} \right)\] \[\left( \frac{x}{V} \right)\] \[\left( \frac{x}{V} \right)\] |
|
Mole fraction |
\[\left( \frac{1-x}{2} \right)\] \[\left( \frac{1-x}{2} \right)\] \[\frac{2x}{2}\] |
\[\frac{1-x}{2\,\left( 2-x \right)}\]\[\frac{3}{2}\left( \frac{1-x}{2-x} \right)\]\[\frac{x}{(2-x)}\] |
\[\left( \frac{2-2x}{3-x} \right)\] \[\left( \frac{1-x}{3-x} \right)\,\,\ \ \left( \frac{2x}{3-x} \right)\] |
\[\left( \frac{1-x}{1+x} \right)\] \[\left( \frac{x}{1+x} \right)\] \[\left( \frac{x}{1+x} \right)\] |
|
Partial pressure |
\[p\,\left( \frac{1-x}{2} \right)\]\[p\,\left( \frac{1-x}{2} \right)\] \[p\,\left( \frac{2x}{2} \right)\] |
\[P\left( \frac{1-x}{2(2-x)\_} \right)\,P\,\left( \frac{3(1-x)}{2(2-x)} \right)\,\frac{Px}{(2-x)}\] |
\[P\,\left( \frac{2-2x}{3-x} \right)\] \[P\left( \frac{1-x}{3-x} \right)\] \[P\,\left( \frac{2x}{3-x} \right)\] |
\[P\left( \frac{1-x}{1+x} \right)\] \[P\left( \frac{x}{1+x} \right)\] \[P\left( \frac{x}{1+x} \right)\] |
|
\[{{K}_{c}}\]
|
\[\frac{4{{x}^{2}}}{\left( 1-x \right){{\,}^{2}}}\] |
\[\frac{4{{x}^{2}}{{V}^{2}}}{27\,\,\left( 1-x \right){{\,}^{4}}}\] |
\[\frac{{{x}^{2}}V}{\left( 1-x \right){{\,}^{3}}}\] |
\[\frac{{{x}^{2}}}{\left( 1-x \right)\,V}\] |
|
\[{{K}_{p}}\] |
\[\frac{4{{x}^{2}}}{\left( 1-x \right){{\,}^{2}}}\] |
\[\frac{16{{x}^{2}}\,\left( 2-x \right){{\,}^{2}}}{27\left( 1-x \right){{\,}^{4}}{{P}^{2}}}\] |
\[\frac{{{x}^{2}}\,\left( 3-x \right)\,}{P\,\left( 1-x \right){{\,}^{3}}}\] |
\[\frac{P{{x}^{2}}}{\left( 1-{{x}^{2}} \right)\,}\] |
Heterogeneous equilibria and equation for equilibrium constant (Equilibrium pressure is P atm)
|
|
\[N{{H}_{4}}HS(s)\]?\[N{{H}_{3}}(g)\] + \[{{H}_{2}}S(g)\] |
\[C(s)+C{{O}_{2}}(g)\]?\[2CO(g)\] |
\[N{{H}_{2}}C{{O}_{2}}N{{H}_{4}}(s)\]?\[2N{{H}_{3}}(g)+C{{O}_{2}}(g)\] |
|
Initial mole |
1 0 0 |
1 1 0 |
1 0 0 |
|
Mole at equilibrium |
(1?x) x x |
(1?x) (1?x) 2x |
(1?x) 2x x |
|
Total moles at equilibrium (solid not included) |
2x |
(1+x) |
3x |
|
Mole fraction |
\[\frac{x}{2x}=\frac{1}{2}\] \[\frac{1}{2}\] |
\[\left( \frac{1-x}{1+x} \right)\] \[\left( \frac{2x}{1+x} \right)\] |
\[\frac{2}{3}\] \[\frac{1}{3}\] |
|
Partial pressure |
\[\frac{P}{2}\] \[\frac{P}{2}\] |
\[P\left( \frac{1-x}{1+x} \right)\] \[P\left( \frac{2x}{1+x} \right)\] |
\[\frac{2P}{3}\] \[\frac{P}{3}\] |
|
|
\[\frac{{{P}^{2}}}{4}\] |
\[\frac{4{{P}^{2}}{{x}^{2}}}{(1-{{x}^{2}})}\] |
\[\frac{4{{P}^{3}}}{27}\] |
(7) Equilibrium constant and standard free energy change : Standard free energy change of a reaction and its equilibrium constant are related to each other at temperature T by the following relation,
\[\Delta {{G}^{o}}=-2.303\,RT\,\log K\]
when, \[\Delta {{G}^{o}}=-ve\], the value of equilibrium constant will be large positive quantity and
when, \[\Delta {{G}^{o}}=+ve\], the value of equilibrium constant is less than 1 i.e., low concentration of products at equilibrium state.
Examples Based on Equilibrium Constant, \[{{K}_{p}}\And {{K}_{c}}\] and \[\Delta {{G}^{o}}=2.303\text{ }RT\text{ }log\text{ }K\]
Example : 1 In the reversible reaction A + B ? C + D, the concentration of each C and D at equilibrium was 0.8 mole/litre, then the equilibrium constant \[{{K}_{c}}\] will be [MP PET 1986]
(a) 6.4 (b) 0.64 (c) 1.6 (d) 16.0
Solution: (d) Suppose 1 mole of A and B each taken then 0.8 mole/litre of C and D each formed remaining concentration of A and B will be (1 – 0.8) = 0.2 mole/litre each.
\[{{K}_{c}}=\frac{[C][D]}{[A][B]}=\frac{0.8\times 0.8}{0.2\times 0.2}=16.0\]
Example : 2 For the system \[A\left( g \right)+2B\left( g \right)\rightleftharpoons C\left( g \right),\] the equilibrium concentrations are (A) 0.06 mole/litre (B) 0.12 mole/litre (C) 0.216 mole/litre. The \[{{K}_{eq}}\] for the reaction is [CPMT 1983]
(a) 250 (b) 416 (c) \[4\times {{10}^{-3}}\] (d) 125
Solution: (a) For reaction\[A+2B\rightleftharpoons C;\] \[{{K}_{eq}}=\frac{[C]}{[A]{{[B]}^{2}}}=\frac{0.216}{0.06\times 0.12\times 0.12}=250\]
Example : 3 Molar concentration of \[{{O}_{2}}\] is 96 gm, it contained in 2 litre vessel, active mass will be
(a) 16 mole/litre (b) 1.5 mole/litre (c) 4 mole/litre (d) 24 mole/litre
Solution: (b) Active mass \[=\frac{\frac{\text{weight}}{\text{M}\text{.wt}\text{.}}}{\text{Volume}}=\frac{\text{weight}}{\text{M}\text{.wt}\text{.}\times \text{Volume}}=\frac{96}{32\times 2}=\frac{3}{2}=1.5\,mol/litre\]
Example : 4 2 moles of \[PC{{l}_{5}}\] were heated in a closed vessel of 2 litre capacity. At equilibrium, 40% of \[PC{{l}_{5}}\] is dissociated into \[PC{{l}_{5}}\] and \[C{{l}_{2}}.\] The value of equilibrium constant is [MP PMT 1989]
(a) 0.266 (b) 0.53 (c) 2.66 (d) 5.3
Solution: (a) At start, \[\underset{2}{\mathop{PC{{l}_{5}}}}\,\]? \[\underset{0}{\mathop{PC{{l}_{3}}}}\,+\underset{0}{\mathop{C{{l}_{2}}}}\,\]
At equilibrium. \[\frac{2\times 60}{100}\] \[\frac{2\times 40}{100}\] \[\frac{2\times 40}{100}\]
Volume of cantainer = 2 litre
\[\therefore \] \[{{K}_{c}}=\frac{\frac{2\times 40}{100\times 2}\times \frac{2\times 40}{100\times 2}}{\frac{2\times 60}{100\times 2}}\] = 0.266
Example : 5 A mixture of 0.3 mole of \[{{H}_{2}}\] and 0.3 mole of \[{{I}_{2}}\] is allowed to react in a 10 litre evacuated flask at \[{{500}^{o}}C\]. The reaction is \[{{H}_{2}}+{{I}_{2}}\rightleftharpoons 2HI\] the \[{{K}_{c}}\] is found to be 64. The amount of unreacted \[{{I}_{2}}\] at equilibrium is
[KCET 1990]
(a) 0.15 mole (b) 0.06 mole (c) 0.03 mole (d) 0.2 mole
Solution: (b) \[{{K}_{c}}=\frac{{{[HI]}^{2}}}{[{{H}_{2}}][{{I}_{2}}]}\]; \[64=\frac{{{x}^{2}}}{0.03\times 0.03}\]
\[{{x}^{2}}=64\times 9\times {{10}^{-4}}\]; \[x=8\times 3\times {{10}^{-2}}=0.24\]
\[x\] is the amount of \[HI\] at equilibrium. Amount of \[{{I}_{2}}\] at equilibrium will be
\[0.30-0.24=0.06\]mole
Example : 6 The rate constant for forward and backward reactions of hydrolysis of ester are \[1.1\times {{10}^{-2}}\] and \[1.5\times {{10}^{-3}}\] per minute respectively. Equilibrium constant for the reaction,
\[C{{H}_{3}}COO{{C}_{2}}{{H}_{5}}+{{H}_{2}}O\]? \[C{{H}_{3}}COOH+{{C}_{2}}{{H}_{5}}OH\] is [AIIMS 1999]
(a) 4.33 (b) 5.33 (c) 6.33 (d) 7.33
Solution: (d) \[{{K}_{f}}=1.1\times {{10}^{-2}}\], \[{{K}_{b}}=1.5\times {{10}^{-3}}\]; \[{{K}_{c}}=\frac{{{K}_{f}}}{{{K}_{b}}}=\frac{1.1\times {{10}^{-2}}}{1.5\times {{10}^{-3}}}=7.33\]
Example: 7 For the reaction \[PC{{l}_{3}}(g)+C{{l}_{2}}(g)\]?\[PC{{l}_{5}}\] at \[{{250}^{o}}C\], the value of \[{{K}_{c}}\] is 26, then the value of \[{{K}_{p}}\] on the same temperature will be [MNR 1990; MP PET 2001]
(a) 0.61 (b) 0.57 (c) 0.83 (d) 0.46
Solution: (a) \[\Delta {{n}_{g}}=12=1\]
\[\therefore \,\,\,{{K}_{p}}={{K}_{c}}{{(RT)}^{\Delta n}}\]; \[\because \,\,\,{{K}_{p}}={{K}_{c}}{{(RT)}^{-1}}\]
Since R = 0.0821 litre\[atm\,{{k}^{1}}mo{{l}^{1}}\], \[T={{250}^{o}}C=250+273=523\]\[{{K}_{p}}=26{{(0.0821\times 523)}^{-1}}=0.61\]
Example : 8 If \[{{K}_{p}}\] for reaction \[{{A}_{(g)}}+2{{B}_{(g)}}\]? \[3{{C}_{(g)}}+{{D}_{(g)}}\] is 0.05 atm at 1000K its \[{{K}_{c}}\] in term of R will be [CBSE PMT 1989]
(a) \[\frac{5\times {{10}^{-4}}}{R}\] (b) \[\frac{5}{R}\] (c) \[\frac{5\times {{10}^{-5}}}{R}\] (d) None of these
Solution: (c) \[{{K}_{p}}={{K}_{c}}{{(RT)}^{\Delta n}}\Rightarrow 5\times {{10}^{-2}}={{K}_{c}}{{(R\times 1000)}^{1}}\Rightarrow {{K}_{c}}=\frac{5\times {{10}^{-5}}}{R}\]
Example : 9 If the equilibrium constant of the reaction \[2HI\rightleftharpoons {{H}_{2}}+{{I}_{2}}\] is 0.25, then the equilibrium constant of the reaction \[{{H}_{2}}+{{I}_{2}}\rightleftharpoons 2HI\] would be [MP PMT 1989, 95]
(a) 1.0 (b) 2.0 (c) 3.0 (d) 4.0
Solution: (d) \[{{K}_{c}}\] for the IInd reaction is reverse of Ist for reaction \[2HI\]? \[{{H}_{2}}+{{I}_{2}}\] is \[0.25{{{K}'}_{c}}\] for reaction, \[{{H}_{2}}+{{I}_{2}}\] ? \[2HI\] will be \[{{{K}'}_{c}}=\frac{1}{{{K}_{c}}}=\frac{1}{0.25}=4\].
Example : 10 If equilibrium constant for reaction 2AB ?\[{{A}_{2}}+{{B}_{2}}\], is 49, then the equilibrium constant for reaction AB ? \[\frac{1}{2}{{A}_{2}}+\frac{1}{2}{{B}_{2}}\], will be [MP PMT 2003; EAMCET 1998]
(a) 7 (b) 20 (c) 49 (d) 21
Solution: (a) 2AB ?\[{{A}_{2}}+{{B}_{2}}\]
\[{{K}_{c}}=\frac{[{{A}_{2}}][{{B}_{2}}]}{{{[AB]}^{2}}}=49\]
For reaction AB ? \[\frac{1}{2}{{A}_{2}}+\frac{1}{2}{{B}_{2}}\]
\[{{{K}'}_{c}}=\frac{{{[{{A}_{2}}]}^{1/2}}{{[{{B}_{2}}]}^{1/2}}}{[AB]}\]
\[{{{K}'}_{c}}=\sqrt{{{K}_{c}}}=\sqrt{49}=7\]
Example : 11 For the reaction \[2N{{O}_{2(g)}}\] ? \[2N{{O}_{(g)}}+{{O}_{2(g)}}\] \[{{K}_{c}}=1.8\times {{10}^{-6}}\] at \[{{185}^{o}}C\]. At \[{{185}^{o}}C\], the value of \[{{{K}'}_{c}}\] for the reaction \[N{{O}_{(g)}}+\frac{1}{2}{{O}_{2(g)}}\]? \[N{{O}_{2(g)}}\] is [UPSEAT 2000]
(a) \[0.9\times {{10}^{6}}\] (b) \[7.5\times {{10}^{2}}\] (c) \[1.95\times {{10}^{-3}}\] (d) \[1.95\times {{10}^{3}}\]
Solution: (b) Reaction is reversed and halved.
\[\therefore \,\,\,{{{K}'}_{c}}=\frac{1}{\sqrt{{{K}_{c}}}}\]; \[{{{K}'}_{c}}=\frac{1}{\sqrt{1.8\times {{10}^{-6}}}}\] =\[7.5\times {{10}^{2}}\]
Example : 12 In an equilibrium reaction for which \[\Delta {{G}^{0}}=0\] the equilibrium constant \[{{K}_{p}}\] should be [BHU 1987]
(a) 0 (b) 1 (c) 2 (d) 10
Solution: (b) If \[\Delta {{G}^{0}}=0\] and \[\Delta {{G}^{0}}=-2.303RT\ \log {{K}_{p}}\]
\[\log \ {{K}_{p}}=0\], \[{{K}_{p}}=1\]
Example : 13 \[\Delta {{G}^{0}}\] for \[H{{I}_{(g)}}\cong +1.7kJ/mole\] what is the equilibrium constant at \[{{25}^{o}}C\] for \[2H{{I}_{(g)}}\] ? \[{{H}_{2(g)}}+{{I}_{2(g)}}\]
[KCET 1992]
(a) 24.0 (b) 3.9 (c) 2.0 (d) 0.5
Solution: (d) \[\Delta {{G}^{0}}=-2.303RT\ \log {{K}_{p}}=-2.303\times 8.314\times {{10}^{-3}}\times 298\ \log \ {{K}_{p}}\]
\[1.7=-2.303\times 8.314\times {{10}^{-3}}\times 298\ \log {{K}_{p}}\]
\[{{K}_{p}}=0.5\]
Example : 14 It is found that the equilibrium constant increases by a factor of four when the temperature is increased from \[{{25}^{o}}C\] to \[{{40}^{o}}C\]. The value of \[\Delta {{H}^{o}}\] is
(a) \[25.46\,kJ\,mo{{l}^{-1}}\] (b) \[171.67\,kJ\,mo{{l}^{-1}}\] (c) \[89.43\,kJ\,mo{{l}^{-1}}\] (d) \[71.67\,kJ\,mo{{l}^{-1}}\]
Solution: (d) Using the equation,
\[\log \frac{{{({{K}_{p}})}_{{{40}^{o}}C}}}{{{({{K}_{p}})}_{{{25}^{o}}C}}}=\frac{\Delta H}{2.303R}\left( \frac{1}{{{T}_{1}}}-\frac{1}{{{T}_{2}}} \right)\],
we get \[\log 4=\frac{\Delta H}{2.303\times 8.314}\left( \frac{1}{273+25}-\frac{1}{273+40} \right)\]
\ \[\Delta H=71.67\,kJ\,mo{{l}^{-1}}\]
Example : 15 \[{{K}_{c}}\] for the reaction \[A\,(g)+B\,(g)\] ? 2C(g) is 3.0 at 400K. In an experiment “a” mol of A is mixed with 3 mol of B in a 1-L vessel. At equilibrium 3 mol of C is formed. The value of ‘a’ will be,
(a) 4.5 mol (b) 9.5 mol (c) 2.5 mol (d) 3.5 mol
Solution: (d) \[\underset{a-x}{\mathop{\underset{a}{\mathop{A(g)}}\,}}\,\,+\underset{3-x}{\mathop{\underset{3}{\mathop{B(g)}}\,}}\,\] ? \[\underset{2x}{\mathop{\underset{0}{\mathop{2C(g)}}\,}}\,\]
From the equation, \[2x=3\Rightarrow x=1.5\]
\[{{K}_{c}}=\frac{4{{x}^{2}}}{(a-x)(b-x)}\]; \[3=\frac{4\times {{(1.5)}^{2}}}{(a-1.5)(3-1.5)}\]; \[3=\frac{4\times 2.25}{(a-1.5)(1.5)}\] Þ a = 3.5
Example : 16 For the reaction \[AB(g)\] ? \[A(g)+B(g)\], AB is 33% dissociated at a total pressure of P. Then
(a) \[P={{K}_{p}}\] (b) \[P=4{{K}_{p}}\] (c) \[P=3{{K}_{p}}\] (d) \[p=8{{k}_{p}}\]
Solution: (d) \[\underset{2/3}{\mathop{\underset{-1/3}{\mathop{\underset{1}{\mathop{AB(g)}}\,}}\,}}\,\] ? \[\underset{1/3}{\mathop{\underset{+1/3}{\mathop{\underset{0}{\mathop{A(g)}}\,}}\,}}\,\,+\,\underset{1/3}{\mathop{\underset{+1/3}{\mathop{\underset{0}{\mathop{B(g)}}\,}}\,}}\,\]
\[{{\left( \sum n \right)}_{eq/m}}=\frac{2}{3}+\frac{1}{3}+\frac{1}{3}=\frac{4}{3}\]
\[{{k}_{p}}=\frac{{{P}_{A}}{{P}_{B}}}{{{P}_{AB}}}=\frac{\frac{1/3}{4/3}P\frac{1/3}{4/3}P}{\frac{2/3}{4/3}P}=\frac{1}{8}P\]
\[P=8{{K}_{p}}\]
Example : 17 The total pressure observed at equilibrium in the dissociation of solid ammonium carbamate at a certain temperature is 2.0 atm. The equilibrium constant \[{{K}_{p}}\] is
(a) 4.185 (b) 1.185 (c) 2.276 (d) 1.072
Solution: (b) \[N{{H}_{4}}COON{{H}_{2}}(s)\] ? \[\underset{\frac{2}{3}P}{\mathop{2N{{H}_{3}}(g)}}\,\,+\,\underset{\frac{1}{3}P}{\mathop{C{{O}_{2}}(g)}}\,\]
\[{{K}_{p}}=P_{N{{H}_{3}}}^{2}{{P}_{C{{O}_{2}}}}={{\left( \frac{2}{3}P \right)}^{2}}\left( \frac{1}{3}P \right)=\frac{4}{27}{{P}^{3}}=\frac{4}{27}\times {{(2)}^{3}}=1.185\]
Example : 18 At the equilibrium of the reaction \[{{N}_{2}}{{O}_{4}}(g)\] ? \[2N{{O}_{2}}(g)\], the observed molar mass of \[{{N}_{2}}{{O}_{4}}\] is 77.70 g. The percentage dissociation of \[{{N}_{2}}{{O}_{4}}\] is
(a) 28.4 (b) 46.7 (c) 22.4 (d) 18.4
Solution: (d) \[\alpha =\frac{{{M}_{Th}}-{{M}_{obs}}}{{{M}_{obs}}(n-1)}\]; Molar mass of \[{{N}_{2}}{{O}_{4}}=92\,g\,mo{{l}^{-1}}\]
Here, n = 2; \[\alpha =\frac{92.00-77.70}{77.70(2-1)}=0.184=18.4%\]
Example : 19 In the equilibrium \[{{H}_{2}}O(g)\] ? \[{{H}_{2}}(g)\,+\frac{1}{2}{{O}_{2}}(g)\], the extent of dissociation of water when p = 1 atm and \[K=2.08\times {{10}^{-3}}\] is approximately
(a) 2% (b) 0.2% (c) 20% (d) 1%
Solution: (a) For the equilibrium \[{{H}_{2}}O\,(g)\to {{H}_{2}}(g)+\frac{1}{2}{{O}_{2}}(g)\]
\[{{K}_{p}}=\frac{{{\alpha }^{3/2}}{{P}^{1/2}}}{\sqrt{2}}P=1\,atm\]
\[\alpha ={{(\sqrt{2}.{{K}_{p}})}^{2/3}}=0.0205\approx 2%\]
You need to login to perform this action.
You will be redirected in
3 sec
