Bonding and hybridisation inorganic compounds
Category : NEET
Bonding and hybridisation in organic compounds
Bonding in organic compounds.
The organic compounds are carbon compounds consisting of one or more carbon atoms. Carbon must form only covalent bonds, i.e., it should share its valency electrons with other atoms.
According to the modern concept, a covalent bond is formed between two atoms if there is an overlapping of an atomic orbital of one atom with an atomic orbital of another atom. The overlapping is possible by two ways,
(1) End to end overlapping: This type of overlapping is possible between \[s-s,\,s-{{p}_{x}}\] and \[{{p}_{x}}-{{p}_{x}}\]atomic orbitals. The molecular bond formed is termed as sigma \[(\sigma )\] bond.
(2) Sidewise or parallel or lateral overlapping: Such overlapping is possible between \[p-p\] atomic orbitals. The molecular bond formed is termed as \[pi\,(\pi )\] bond.
|
\[\sigma \]-Bond |
|
\[\pi \]-Bond |
|
Formed by End to End overlap of AO?s. |
|
Formed by lateral overlap of \[p\]-orbitals. |
|
Has cylindrical charge symmetry about bond axis. |
|
Has maximum charge density in the cross-sectional plane of the orbitals. |
|
Has free rotation |
|
No free rotation, i.e., frozen rotation |
|
Low energy |
|
Higher energy |
|
Only one s bond can exist between two atoms, |
|
One or two p bonds can exist between two atoms. |
|
Sigma bonds are directional. Thus the geometry of the molecule depends on the s bonds. |
|
p bonds are not directional. Geometry of the molecule not depends on p bond. |
|
Area of overlapping is higher hence bond is stronger. |
|
Area of overlapping is small hence bond is weaker. |
|
s bond can have independent existance. |
|
p bond always exist along with a s bond and p bond is formed after the formation of s bond. |
![]() Hybridisation in Organic Compounds.
Hybridisation in Organic Compounds.
(1) The process of mixing atomic orbitals to form a set of new equivalent orbitals is termed as hybridisation. There are three types of hybridisation encountered in carbon atom. These are,
(i) \[s{{p}^{3}}\] hybridisation (involved in saturated organic compounds containing only single covalent bonds),
(ii) \[s{{p}^{2}}\] hybridisation (involved in organic compounds having carbon linked by double bonds) and
(iii) \[sp\] hybridisation (involved in organic compounds having carbon linked by a triple bonds).
|
Type of hybridisation |
Number of orbitals used |
Number of unused p-orbitals |
Bond |
Bond angle |
Geometry |
% s-character |
|
sp3 |
1s and 3p |
Nil |
Four -s |
109.5° |
Tetrahedral |
25 or 1/4 |
|
sp2 |
1s and 2p |
One |
Three -s One -p |
120° |
Trigonal |
33.33 or 1/3 |
|
sp |
1s and 1p |
Two |
Two -s Two -p |
180° |
Linear |
50 or 1/2 |
(2) Determination of hybridisation at different carbon atoms: It can be done by two methods,
(i) First method: In this method hybridisation can be know by the number of \[\pi -\] bonds present on that particular atom.
|
Number of p? bond/s |
0 |
1 |
2 |
|
Type of hybridisation |
sp3 |
sp2 |
sp |
Examples: (i) \[\underset{_{s{{p}^{3}}}^{\downarrow }\,\,}{\mathop{C{{H}_{3}}}}\,-\underset{_{s{{p}^{2}}}^{\downarrow }}{\mathop{CH}}\,=\underset{_{s{{p}^{2}}}^{\downarrow }}{\mathop{CH}}\,\underset{_{s{{p}^{2}}}^{\ \downarrow }}{\overset{\ _{|\,|}^{O}}{\mathop{-C\,-}}}\,\underset{_{s{{p}^{3}}}^{\downarrow }\,\,}{\mathop{C{{H}_{3}}}}\,\] (ii) \[\underset{_{s{{p}^{2}}}^{\downarrow \ }}{\mathop{C{{H}_{2}}}}\,=\underset{_{sp}^{\,\downarrow }}{\mathop{C}}\,=\underset{_{s{{p}^{2}}}^{\downarrow }}{\mathop{C{{H}_{2}}}}\,\]
(iii) \[\underset{_{s{{p}^{3}}}^{\downarrow }}{\mathop{\,\,\,C{{H}_{3}}}}\,-\underset{_{s{{p}^{2}}}^{\downarrow }}{\mathop{CH}}\,=\underset{_{s{{p}^{2}}}^{\downarrow }}{\mathop{CH}}\,-\underset{_{s{{p}^{3}}}^{\downarrow }\,\,}{\mathop{C{{H}_{2}}}}\,-\underset{\,_{sp}^{\downarrow }}{\mathop{C}}\,\equiv \underset{_{sp}^{\downarrow }}{\mathop{N}}\,\] (iv) \[\underset{\,\,\,\,\,_{sp}^{\downarrow }}{\mathop{HC}}\,\equiv \underset{\,_{sp}^{\downarrow }}{\mathop{C}}\,-\underset{_{s{{p}^{2}}}^{\downarrow }}{\mathop{CH}}\,=\underset{_{s{{p}^{2}}}^{\downarrow }\,\,}{\mathop{C{{H}_{2}}}}\,\]
Note: q In diamond carbon is sp3 hybridised and in graphite carbon is \[s{{p}^{2}}\] hybridised.
q This method cannot be used for those atoms of the molecule which have positive charge, negative charge or odd electron.
(ii) Second method: (Electron pair method)
ep = bp + lp; where ep = electron pair present in hybrid orbitals , bp = bond pair present in hybrid orbitals
Number of bp = Number of atoms attached to the central atom of the species
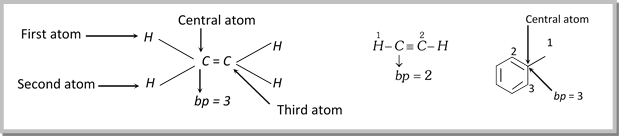
Number of lp’s can be determined as follows,
(a) If carbon has \[\pi \]- bond/s or positive charge or odd electron, than lp on carbon will be zero.
(b) If carbon has negative charge, then lp will be equal to one.
Number of electron pairs (ep) tells us the type of hybridisation as follows,
|
ep |
2 |
3 |
4 |
5 |
6 |
|
Type of hybridisation |
\[sp\] |
\[s{{p}^{2}}\] |
\[s{{p}^{3}}\] |
\[s{{p}^{3}}d\] |
\[s{{p}^{3}}{{d}^{2}}\] |
Example:
(i) \[\underset{\underset{\frac{\,lp=0}{ep=2,\,sp}}{\mathop{bp=2}}\,}{\mathop{C{{H}_{2}}=\underset{\downarrow \,\,\,\,}{\overset{\oplus \,\,\,\,\,}{\mathop{CH}}}\,}}\,\]
(ii) \[\underset{\underset{\frac{\,lp=1}{ep=3,\,s{{p}^{2}}}}{\mathop{bp=2}}\,}{\mathop{C{{H}_{2}}=\underset{\downarrow \,\,\,\,}{\overset{\Theta \,\,\,\,\,}{\mathop{CH}}}\,}}\,\]
(iii) 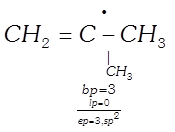
(iv) 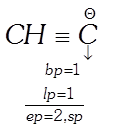
(v) 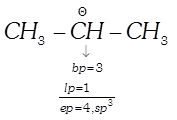
(3) Applications of hybridisation
(i) Size of the hybrid orbitals: Since \[s\]- orbitals are closer to nucleus than \[p\]- orbitals, it is reasonable to expect that greater the \[s\] character of an orbital the smaller it is. Thus the decreasing order of the size of the three hybrid orbitals is opposite to that of the decreasing order of \[s\] orbital character in the three hybrid orbitals.
\[s{{p}^{3}}>s{{p}^{2}}>sp\]
(ii) Electronegativity of different orbitals
(a) Electronegativity of s-orbital is maximum.
(b) Electronegativity of hybrid orbital µ % s-character in hybrid orbitals
\[\underset{\,\,s\text{-character in decreasing order and electronegativity in decreasing order}}{\mathop{\xrightarrow{\ \ \ \ \ \underset{\text{ }\!\!%\!\!\text{ }s\text{ -character}}{\mathop{\text{Orbital}}}\,\text{ }\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\underset{50}{\mathop{\,sp}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\underset{33.33}{\mathop{\,\,s{{p}^{2}}}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\underset{25}{\mathop{\,\,\,s{{p}^{3}}}}\,\ \ \ \ \ \ \ \ \ \ \ \ }}}\,\]
Thus sp-hybrid carbon is always electronegative in character and \[s{{p}^{3}}\]- hybrid carbon is electropositive in character. \[s{{p}^{2}}\]-hybrid carbon can behave as electropositive (in carbocation) as well as electronegative (in carbanion) in characte \[C{{H}_{3}}-\overset{\oplus \,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,\] \[C{{H}_{2}}=\overset{\oplus \,\,\,\,\,\,\,\,}{\mathop{CH}}\,\]


(c) Electronegativity of different hybrid and unhybrid orbitals in decreasing order is as follows
\[\underset{\text{ }\!\!%\!\!\text{ s-character in decreasing order and}\ \text{electronegativity in decreasing order}\text{.}}{\mathop{\xrightarrow{\,\,\,\,\,\,\,\,\,\,\,\,\,\,\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ s>sp>s{{p}^{2}}>s{{p}^{3}}>p\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \,\,\,\,\,\,\,\,\,\,\,\,}}}\,\]
(iii) Bond length variation in hydrocarbons
% s orbital character \[\propto \frac{1}{C-C\,\text{bond length}}\propto \frac{1}{C-H\text{ bond length}}\]
|
Bond type (C ? H) |
Bond length |
Bond type (C ? C) |
Bond length |
|
\[s{{p}^{3}}-s\] (alkanes) |
1.112Å |
\[s{{p}^{3}}-s{{p}^{3}}\](alkanes) |
1.54 Å |
|
\[s{{p}^{2}}-s\] (alkenes) |
1.103Å |
\[s{{p}^{2}}-s{{p}^{2}}\](alkenes) |
1.34Å |
|
\[sp-s\] (alkynes) |
1.08Å |
\[sp-sp\](alkynes) |
1.20Å |
Note: q C–C bond length in benzene lies between single and double bond due to resonance. (1.40Å).
(iv) Bond strength in hydrocarbons: The shorter the bond, the greater the compression between atomic nuclei and hence greater the strength of that bond is. Thus the bond formed by \[sp\] hybridised carbon is strongest (i.e., it has maximum bond energy) while that formed by \[s{{p}^{3}}\] hybridised carbon is the weakest (i.e., it has minimum bond energy). This is evident by the bond energies of the various types of \[C-H\] and \[C-C\] bonds.
|
Bond type (C ? H) |
Bond energy
(kcal/mole) |
Bond type (C ? C) |
Bond energy (kcal/mole) |
|
\[s{{p}^{3}}-s\] (in alkanes) |
104 |
\[s{{p}^{3}}-s{{p}^{3}}\](in alkanes) |
80 ? 90 |
|
\[s{{p}^{2}}-s\] (in alkenes) |
106 |
\[s{{p}^{2}}-s{{p}^{2}}\](in alkenes) |
122 ? 164 |
|
\[sp-s\] (in alkynes) |
121 |
\[sp-sp\](in alkynes) |
123 ? 199 |
(v) Acidity of hydrocarbons
(a) Hydrogen present on electronegative carbon is acidic in character.
(b) Acidity of hydrogen is directly proportional to the electronegativity of atom on which hydrogen is present.
Thus
\[\underset{\begin{smallmatrix}
\text{Electronegativity of atom in decreasing order} \\
\text{Acidity of compounds in deceasing order }
\end{smallmatrix}}{\mathop{\xrightarrow{H-O-H N{{H}_{3}} CH\equiv CH}}}\,\]
(c) Acidity of hydrocarbon µ % s-character
\[CH\equiv CH\] \[C{{H}_{2}}=C{{H}_{2}}\] \[C{{H}_{3}}-C{{H}_{3}}\]
% s-character 50 33.33 25
\[pKa\] 25 44 50
s- character in decreasing order and acidity in decreasing order
Note: q Acidity µ Ka and Acidity \[\propto \frac{1}{pKa}(pKa=-\log Ka)\]
q Order of acidic nature of alkynes is, \[HC\equiv CH>HC\equiv C-C{{H}_{3}}\]
q The relative acidic character follows the order;
\[{{H}_{2}}O>ROH>HC\equiv CH>N{{H}_{3}}>C{{H}_{2}}\equiv C{{H}_{2}}>C{{H}_{3}}-C{{H}_{3}}\]
Obviously, the basic character of their conjugate bases follows the reverse order, i.e.,
\[C{{H}_{3}}CH_{2}^{}>C{{H}_{2}}=C{{H}^{}}>NH_{2}^{}>HC\equiv {{C}^{}}>R{{O}^{}}>H{{O}^{}}\]
You need to login to perform this action.
You will be redirected in
3 sec
