Notes - Dipole moment, Resonance and reaction intermediates
Category : NEET
Dipole moment, resonance and reaction intermediates
Hybridisation in Organic Compounds
(1) Due to differences in electronegativity polarity developes between two adjacent atoms in the molecule (i.e., in a bond). The degree of polarity of a bond is called dipole moment. Dipole moment is represented by \[\mu \] and its unit is Debye (D).
\[\mu =e\times l\]
Where, \[e=\] magnitued of separated charge in e.s.u., \[l=\]internuclear distance between two atoms i.e., bond length in cm.
The dipole moment is denoted by arrow head pointing towards the positive to the negative end (?).
(2) Dipole moment of the compound does not depend only on the polarity of the bond but also depends on the shape of the molecule.Dipole moment of symmetrical compound is always zero, (\[\mu =0\]). Symmetrical compounds are those compounds which fulfil following two conditions,
(i) Central atom is bonded with the same atoms or groups.Examples:
\[\underset{\text{Symmetrical molecules}}{\mathop{{{H}_{2}},B{{F}_{3}},C{{S}_{2}},C{{H}_{2}}=C{{H}_{2}},CH\equiv CH}}\,\]
(ii) Central atom should have no lone pair of electrons.
Examples: \[\underset{\text{Symmetrical molecules}}{\mathop{CC{{l}_{4}},\,C{{H}_{4}},B{{H}_{3}},C{{O}_{2}}}}\,\] \[\underset{\text{Unsymmetrical molecules}}{\mathop{{{H}_{2}}\overset{.\,\,.}{\mathop{O}}\,,\,\,\,\,{{H}_{2}}\overset{.\,\,.}{\mathop{S}}\,}}\,\]
Note: q Compounds which have regular tetrahedral structure has no dipole moment.
(3) \[\mu \propto \]electronegativity of central atom or surrounding atoms present on the central atom of the molecule.
\[\underset{\begin{smallmatrix}
\text{Electronegativity in decreasing order} \\
\mu \text{ is also in decreasing order }
\end{smallmatrix}}{\mathop{\xrightarrow{CH{{F}_{3}} CHC{{l}_{3}} CHB{{r}_{3}} CH{{I}_{3}}}}}\,\]
\[\underset{\begin{smallmatrix}
\text{Electronegativity of central atom is in decreasing } \\
\text{order }\mu \text{ is also in decreasing order }
\end{smallmatrix}}{\mathop{\xrightarrow{\,\,\,\,N{{H}_{3}} P{{H}_{3}} As{{H}_{3}} Sb{{H}_{3}}\ \ \ \ }}}\,\]
Note: q Decreasing order of dipole moment in \[C{{H}_{3}}Cl,\,C{{H}_{2}}C{{l}_{2}},\,CHC{{l}_{3}}\] and \[CC{{l}_{4}}\] is
\[C{{H}_{3}}Cl>C{{H}_{2}}C{{l}_{2}}>CHC{{l}_{3}}>CC{{l}_{4}}\]
m = 1.86 D 1.62 D 1.03 0
q Alkynes has larger dipole moment because the electronegativity of \[sp-C\] is more than that of \[s{{p}^{2}}-C\].
(4) \[\mu \] cis \[>\,\mu \] trans in geometrical isomers.
(5) Dipole moment of the trans derivative of the compound \[(a)(b)C=C(a)(b)\] will only be zero if both \[a\] and \[b\] will be in the form of atoms.
Example: 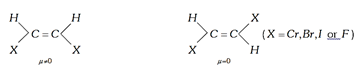
If both will not be atoms then \[\mu \] trans may or may not be zero.
If group have non-linear moments, then the dipole moment of the trans isomer will not be zero. If group have linear moments, then the dipole moment of the trans isomer will be zero.
Example: 
(6) Dipole moment of disubstituted benzene
(i) When both groups \[X\] and \[Y\] are electron donating or both groups are electron with drawing

Then, \[\mu =\sqrt{\mu _{1}^{2}+\mu _{2}^{2}+2{{\mu }_{1}}{{\mu }_{2}}\cos \theta }\]
Where, \[{{\mu }_{1}}=\] dipole moment of bond\[C-X\], \[{{\mu }_{2}}=\] dipole moment of bond \[C-Y\], \[\theta =\] angle between \[X\]and \[Y.\]
If value of \[\theta \] will be more, then \[\cos \theta \] will be less. Hence, dipole moment will be as,
\[\underset{\mu \text{ in decreasing order}}{\mathop{\xrightarrow{o-\text{derivative }>\text{ }m\text{-derivative }>\text{ }p\text{-derivative }}}}\,\]
(ii) When one group is electron with drawing and the other group is electron donating then,
\[\mu =\sqrt{\mu _{1}^{2}+\mu _{2}^{2}-2{{\mu }_{1}}{{\mu }_{2}}\cos \theta }\]
Hence, dipole moment is as follows,
\[\underset{\mu \text{ in decreasing order}}{\mathop{\xrightarrow{o-\text{derivative }>\text{ }\,\,m\text{-derivative }>\text{ }p\text{-derivative }}}}\,\]
![]() Reaction Intermediates.
Reaction Intermediates.
Short lived fragments called reaction intermediates result from homolytic and heterolytic bond fission. The important reaction intermediates are free radicals, carbocations, carbonions, carbenes, benzyne and nitrenes.
Free radicals
A free radical may be defined as an atom or group of atoms having an odd or unpired electron. These result on account of homolytic fission of a covalent bond and are denoted by putting a dot (.) against the symbol of atom or group of atoms.
\[\underset{\begin{smallmatrix}
\text{Chlorine} \\
\text{free}\,\text{radical}
\end{smallmatrix}}{\mathop{\overset{.}{\mathop{Cl}}\,}}\,;\ \ \ \ \ \ \underset{\begin{smallmatrix}
\text{Hydrogen} \\
\text{free}\,\text{radical}
\end{smallmatrix}}{\mathop{\overset{.}{\mathop{H}}\,}}\,;\ \ \ \ \ \ \underset{\begin{smallmatrix}
\text{ Methyl} \\
\text{free}\,\text{radical}
\end{smallmatrix}}{\mathop{{{H}_{3}}\overset{.}{\mathop{C}}\,}}\,;\] \[\underset{\begin{smallmatrix}
\,\,\,\,\,\,\,Ethyl \\
free\,radical
\end{smallmatrix}}{\mathop{{{H}_{3}}C\overset{H}{\mathop{\overset{\,\,|\,\,\,.}{\mathop{-\underset{H\,\,\,}{\mathop{\underset{|\,\,\,\,}{\mathop{C\,\,\,\,}}\,}}\,}}\,}}\,}}\,\]; \[\underset{\begin{smallmatrix}
\text{Triphenyl}\,\text{methyl}\, \\
\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\text{radical}
\end{smallmatrix}}{\mathop{{{C}_{6}}{{H}_{5}}-\overset{{{C}_{6}}{{H}_{5}}}{\mathop{\overset{|\,\,.\,\,\,\,\,}{\mathop{\underset{{{C}_{6}}{{H}_{5}}}{\mathop{\underset{|\,\,\,\,\,\,\,}{\mathop{C\,\,\,\,\,\,\,}}\,}}\,}}\,}}\,}}\,\]
The first stable organic free radical was triphenyl methyl radical and it was prepared by Gomberg.
(1) Characteristics of free radical.
|
(i) The formation of free radical is initiated by heat, light or catalysts. |
|
(a) \[Cl:Cl\underset{\text{sunlight}}{\mathop{\xrightarrow{\text{Energy}}}}\,C\overset{.}{\mathop{l}}\,+C\overset{.}{\mathop{l}}\,\] |
|
(b) \[{{H}_{3}}C:H\xrightarrow{102\,kcal}{{H}_{3}}\overset{.}{\mathop{C}}\,+\overset{.}{\mathop{H}}\,\] |
|
(c) \[{{H}_{3}}C-C{{H}_{2}}-H\xrightarrow{97\,kcal}\underset{\text{(primary)}}{\mathop{{{H}_{3}}C-\overset{.}{\mathop{C}}\,{{H}_{2}}+\overset{.}{\mathop{H}}\,}}\,\] |
|
(d) |
|
(e) |
|
(f) \[C{{H}_{2}}=CH-C{{H}_{2}}-H\xrightarrow{86kcal}\underset{\text{(allyl)}}{\mathop{C{{H}_{2}}=CH-\overset{.}{\mathop{C}}\,{{H}_{2}}+\overset{.}{\mathop{H}}\,}}\,\] |
|
(g) |
|
(h) \[C{{H}_{3}}-N{{H}_{2}}\xrightarrow{heat/light}\overset{.}{\mathop{C}}\,{{H}_{3}}+\overset{.}{\mathop{N}}\,{{H}_{2}}\] |
|
(ii) Free radicals are generally electrically neutral. |
|
(iii) There are seven electrons in the out most orbit of carbon of carbon free radicals. |
|
(iv) Owing to the presence of an odd electron; a carbon radical is paramagnetic in nature. Due to this reason free radicals are highly reactive. |
|
(v) Free radicals are neutral electrophiles. |
|
(vi) Chemical reactions which takes place with the formation of free radical as an intermediate are known as free radical reactions. These reactions takes place in three steps, |
(a) Initiation (b) Propagation and (c) Termination
Each of these steps, in turn, may involve two or more steps.
(a) Initiation step: In this step, homolytic bond fission takes place in the presence of initiator, i.e., peroxide, \[hv\], heat etc. The process is always endothermic.
\[Cl-Cl\underset{\Delta }{\mathop{\xrightarrow{hv\,or}}}\,\overset{.}{\mathop{C}}\,l+\overset{.}{\mathop{C}}\,l\] \[\Delta H=+ve\]
(b) Propagation step: Propagation step is always two or more than two step process and all propagation steps should be exothermic, otherwise free radical reactions would not take place.
Propagation step I: In this step, formation of free radical as reaction intermediate takes place. For example,
\[C{{H}_{3}}-H+\overset{.}{\mathop{C}}\,l\xrightarrow{{}}\overset{.}{\mathop{C}}\,{{H}_{3}}+HCl\] \[\Delta H=\] negative
Propagation step II: Reaction intermediate reacts with reagent to give the product.
![]() \[\Delta H=\] negative
\[\Delta H=\] negative
(c) Termination step : In this step, free radical formed in the last propagation step (generally propagation step II) is destroyed by the addition of free radical or by the addition of some impurities like \[CHC{{l}_{3}}\] or \[CC{{l}_{4}}\].
\[\overset{.}{\mathop{C}}\,l+\overset{.}{\mathop{C}}\,l\xrightarrow{{}}C{{l}_{2}}\]
Reaction velocity of free radical reaction is very-very high.
· Note: Certain compounds, known as inhibitor, retar velocity of free radical reactions. Common inhibitors are\[{{O}_{2}}\], \[{{I}_{2}}\], p-benzoquinone and diphenylamine.
(2) Relative stabilities of free radicals: The tertiary alkyl free radicals are most stable and methyl free radial is least stable, i.e., the free radical formed easily has greater stability.
\[\underset{\begin{smallmatrix}
\,\,\,\text{Tertiary} \\
\text{free}\,\text{radical}
\end{smallmatrix}}{\mathop{R-\underset{R}{\mathop{\underset{|}{\mathop{\overset{R}{\mathop{\overset{|\,.}{\mathop{C}}\,}}\,}}\,}}\,}}\,\,\,\,\,\,>\,\,\,\,\,\,\underset{\begin{smallmatrix}
\text{Secondary} \\
\text{free}\,\text{radical}
\end{smallmatrix}}{\mathop{R-\underset{H}{\mathop{\underset{|}{\mathop{\overset{R}{\mathop{\overset{|\,.}{\mathop{C}}\,}}\,}}\,}}\,}}\,\,\,\,\,\,>\,\,\,\,\,\,\underset{\begin{smallmatrix}
\text{ Primary} \\
\text{free}\,\text{radical}
\end{smallmatrix}}{\mathop{R-\underset{H}{\mathop{\underset{|}{\mathop{\overset{H}{\mathop{\overset{|\,.}{\mathop{C}}\,}}\,}}\,}}\,}}\,\,\,\,\,\,>\,\,\,\,\,\underset{\text{Methyl radical }}{\mathop{H-\underset{H}{\mathop{\underset{|}{\mathop{\overset{H}{\mathop{\overset{|\,.}{\mathop{C}}\,}}\,}}\,}}\,}}\,\]
Benzyl and allyl free radicals are resonance stabilized hence are more stable than alkyl free radicals.
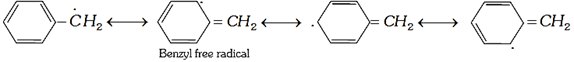
\[C{{H}_{2}}=CH-\overset{.}{\mathop{C}}\,{{H}_{2}}\underset{{}}{\longleftrightarrow}\overset{.}{\mathop{C}}\,{{H}_{2}}-CH=C{{H}_{2}}\] (Allyl)
Relative stability of free radicals are: Benzyl > allyl \[>~3{}^\circ >2{}^\circ >1{}^\circ >\]methyl, vinyl
The stability of various free radicals can be compared from their bond dissociation energies (the energy required for the homolytic cleavage of the covalent bond in the molecule to form two radicals). In general, the smaller the amount of energy required for bond breaking, the more stable is the radical.
(3) Structure of alkyl free radicals: The carbon atom of alkyl free radicals which is bonded to only three atoms or groups of atoms is \[s{{p}^{2}}\] hybridized. Thus, free radicals have a planar structure with odd electron situated in the unused \[p\]-orbital at right angles to the plane of hybrid orbitals.
However, in the substituted alkyl radicals the \['s'\] character of these orbitals has been found to increase, thus, tending to the pyramidal shape.
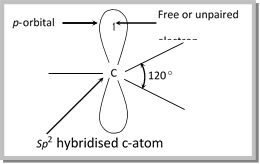
\[\overset{.}{\mathop{C}}\,{{H}_{3}}<\overset{.}{\mathop{C}}\,{{H}_{2}}F<\overset{.}{\mathop{C}}\,H{{F}_{2}}<\overset{.}{\mathop{C}}\,{{F}_{3}}\].
So, \[C{{F}_{3}}\] is essentially pyramidal in shape.
(4) Stereochemistry of free radicals: To learn about the stereochemistry of free radical reaction. We choose the reaction, chlorination of 1-chloro-2-methyl butane. The reaction products are,
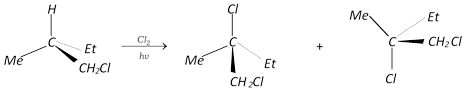
If we review the insight of the reaction, it is clear that the reaction involves racemization at the reaction centre. The free radical should have the \[s{{p}^{2}}\] hybridisation. Obviously a \[C{{l}_{2}}\]molecule could attack either the upper or lower lobe of the \[p\]-orbital with equal chances, leading to racemic mixture.
Intermediate \[\xrightarrow{C{{l}_{2}}}\,\underset{(50%)}{\mathop{d-\text{form}}}\,+\underset{(50%)}{\mathop{l-\text{form}}}\,\]
(5) Reactions involving free radicals,
(i) Chlorination of alkanes (ii) Pyrolysis of alkanes
(iii) Wurtz reaction (iv) Anti-markownikoff rule
(v) Kolbe electrolytic synthesis (vi) Polymerisation initiated by free radical.
Carboniumions (carbocations)
Carbocation is defined as a group of atoms which contain positively charged carbon having only six electrons. It is obtained by heterolytic fission of a covalent bond involving carbon atom. It is denoted by putting a positive charge (+) against the symbol of group of atoms.
(1) Characteristics of carbocations
(i) The formation of carbocations can be done,
![]() (a) By heterolysis of halogen compounds:
(a) By heterolysis of halogen compounds: 
(b) By protonation of alkenes or alcohols: ![]()
![]()
![]()
![]()
(c) By decomposition of Diazo compounds: \[{{C}_{6}}{{H}_{5}}-{{N}_{2}}-Cl\xrightarrow{-Cl}{{C}_{6}}{{H}_{5}}{{\overset{\oplus }{\mathop{N}}\,}_{2}}\xrightarrow{-{{N}_{2}}}\overset{\oplus }{\mathop{{{C}_{6}}}}\,{{H}_{5}}+{{N}_{2}}\]
(ii) Carbocations are not electrically neutral.
(iii) There are six electrons in the out most orbit of carbon of carbocation i.e., 2 electrons less than octet.
(iv) A carbocation is diamagnetic in nature due to all paired electrons.
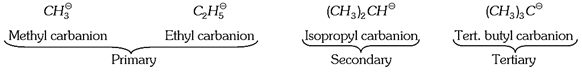
(v) Carbocations are charged electrophiles i.e., they react with nucleophilic reagents. The order of reactivity of carbocations is, Primary \[({{1}^{o}})\] > Secondary \[({{2}^{o}})\] > Tertiary \[({{3}^{o}})\]
(2) Types of carbocations: Carbocations can be classified into the following types,
(i) Alkyl carbocations
(a) When positive charge is present on the alkyl carbon, carbocation is known as alkyl carbocation.
(b) Alkyl carbocation is of four types
\[\underset{\text{Methyl carbocation}}{\mathop{\overset{\oplus }{\mathop{C}}\,{{H}_{3}}}}\,\] \[\underset{\text{Primary alkyl carbocations}}{\mathop{R-\overset{\oplus }{\mathop{C}}\,{{H}_{2}}}}\,\] \[\underset{\text{Secondary alkyl carbocations}}{\mathop{R-\overset{\oplus }{\mathop{C}}\,H-{{R}_{{}}}}}\,\] \[\underset{\text{Tertiary alkyl carbocations}}{\mathop{{{R}_{3}}\overset{\oplus }{\mathop{C}}\,}}\,\]
(c) Stability of alkyl carbocations can be explained by Inductive effect and Hyperconjugation.
(d) According to these two effects the stability order is as follows:
\[R-\overset{\oplus }{\mathop{\underset{R}{\mathop{\underset{|}{\mathop{C}}\,}}\,}}\,-R\] \[R-\overset{\oplus }{\mathop{C}}\,H-R\] \[R-\overset{\oplus }{\mathop{C}}\,{{H}_{2}}\] \[\overset{\oplus }{\mathop{C}}\,{{H}_{3}}\]
Stability in decreasing order
(e) If \[\alpha \]-atom with respect to carbocationic carbon has one or more than one lone pair of electrons then lone pair of electrons strongly stabilises a carbocation due to the delocalisation.
Alkoxy and amino groups are important substituents for such type of carbocations.
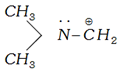 is more stable than \[R-\underset{R}{\mathop{\underset{|}{\mathop{\overset{\oplus }{\mathop{C}}\,}}\,}}\,-R\];
is more stable than \[R-\underset{R}{\mathop{\underset{|}{\mathop{\overset{\oplus }{\mathop{C}}\,}}\,}}\,-R\];
\[C{{H}_{3}}-\underset{.\,\,.}{\mathop{\overset{.\,\,.}{\mathop{O}}\,}}\,-\overset{\oplus }{\mathop{C}}\,{{H}_{2}}\] is more stable than \[R-\underset{R}{\mathop{\underset{|}{\mathop{\overset{\oplus }{\mathop{C}}\,}}\,}}\,-R\]
(ii) Vinyl carbocation: When positive charge is present on vinylic carbon then carbocation is known as vinyl carbocation, \[C{{H}_{2}}=\overset{\oplus }{\mathop{C}}\,H\]
This carbocation is the least stable because positive charge is present on the electronegative carbon.
(iii) Allyl carbocation: \[(C{{H}_{2}}=CH-\overset{\oplus }{\mathop{C}}\,{{H}_{2}})\]
(a) When positive charge is present on the allylic carbon of the allyl group, the carbocation is known as allyl carbocation.
(b) Allyl carbocations are more stable than the alkyl carbocations due to the resonance. Allyl carbocations are stabilised by delocalisation (or resonance).
(c) Allyl carbocations are of the following types,
\[\underset{\text{Primary}}{\mathop{C{{H}_{2}}=CH-\overset{\oplus }{\mathop{C}}\,{{H}_{2}}}}\,\] \[\underset{\text{Secondary}}{\mathop{C{{H}_{2}}=CH-\overset{\oplus }{\mathop{C}}\,H-R}}\,\] \[\underset{\text{Tertiary}}{\mathop{C{{H}_{2}}=CH-\overset{\oplus }{\mathop{\underset{R}{\mathop{\underset{|}{\mathop{C}}\,}}\,}}\,-R}}\,\]
(d) Stability of primary, secondary and tertiary allyl carbocations can be compared by, Inductive effect and Hyperconjugation. Thus, tertiary is more stable than secondary which is more stable than primary allyl carbocation.
(iv) Phenyl methyl carbocations
(a) When positive charge is present on benzyl carbon, carbocation is known as phenyl methyl carbocation.
(b) Phenyl methyl carbocations are of three types,
|
\[{{C}_{6}}{{H}_{5}}-\overset{\oplus }{\mathop{C}}\,{{H}_{2}}\] |
\[{{C}_{6}}{{H}_{5}}-\overset{\oplus }{\mathop{C}}\,H-{{C}_{6}}{{H}_{5}}\] |
\[{{C}_{6}}{{H}_{5}}-\underset{{{C}_{6}}{{H}_{5}}}{\mathop{\underset{|}{\mathop{\overset{\oplus }{\mathop{C}}\,}}\,\,-\,{{C}_{6}}}}\,{{H}_{5}}\] |
|
Benzyl carbocation or Phenyl methyl carbocation (\[{{1}^{o}}\]-carbocation) |
Diphenyl methyl carbocation (\[{{2}^{o}}\]-carbocation) |
Triphenyl methyl carbocation (\[{{3}^{o}}\]-carbocation) |
(c) Stability of phenyl methyl carbocations can be explained by resonance.
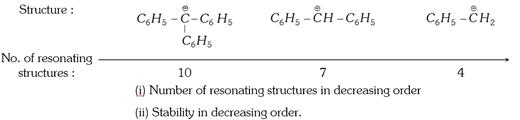
(d) Phenyl methyl carbocations are more stable than allyl carbocations due to the number of resonating structures.
(v) Aromatic carbocations
(a) Cations in which positive charge is present on carbon of aromatic system is known as aromatic carbocation.
(b) Aromatic carbocations are so stable that even their solid states are known. For example tropolium carbocation as tropolium bromide is a yellow solid. It fact tropolium carbocation is about \[{{10}^{11}}\] times more stable than triphenyl methyl carbocation.
(c) Cations obeying Huckel (4n+2) rule are stable because they are aromatic and there is complete delocalisation of positive charge.
(vi) Cyclopropyl methyl carbocations
(a) These carbocations are very stable carbocations. They are more stable than benzyl carbocations.
(b) Stability of cyclopropy methyl carbocations increases with every cyclopropyl group. Thus additional cyclopropyl group has commulative additive effect on the stability. Thus,

Stability in decreasing order
(c) The special stability is a result of conjugation between the bent orbitals of the cyclopropyl ring and the vacant-\[p\]-orbital of the cationic carbon.
(3) Relative: Stability of different types of carbocations in decreasing order
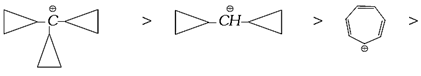
\[{{({{C}_{6}}{{H}_{5}})}_{3}}\overset{\oplus }{\mathop{C}}\,>{{({{C}_{6}}{{H}_{5}})}_{2}}\overset{\oplus }{\mathop{C}}\,H>{{C}_{6}}{{H}_{5}}-\overset{\oplus }{\mathop{C}}\,{{H}_{2}}\ge C{{H}_{2}}=CH-\overset{\oplus }{\mathop{C}}\,{{H}_{2}}\ge \]
\[R-\overset{\oplus }{\mathop{\underset{R}{\mathop{\underset{|}{\mathop{C}}\,}}\,}}\,-R\,\,>R-\overset{\oplus }{\mathop{C}}\,H-R\,\,\,>R-\overset{\oplus }{\mathop{C}}\,{{H}_{2}}\,\,\,>\,\,\overset{\oplus }{\mathop{C}}\,{{H}_{3}}\,\,\,>\,\,C{{H}_{2}}=\overset{\oplus }{\mathop{C}}\,H\]
· Note: More stable is a carbocation, the more easily it is formed.
![]() (4) Structure of carbocation: The carbon atom in carbocation is \[s{{p}^{2}}\] hybridised. The three \[s{{p}^{2}}\] hybrid orbitals form three \[\sigma \] bonds with hydrogen or other carbon atoms of alkyl groups. The unhybridised \[p\]-orbital of carbon remains vacant. Since it involves \[s{{p}^{2}}\] hybridisation, its structure is planar with a bond angle of \[{{120}^{o}}\].
(4) Structure of carbocation: The carbon atom in carbocation is \[s{{p}^{2}}\] hybridised. The three \[s{{p}^{2}}\] hybrid orbitals form three \[\sigma \] bonds with hydrogen or other carbon atoms of alkyl groups. The unhybridised \[p\]-orbital of carbon remains vacant. Since it involves \[s{{p}^{2}}\] hybridisation, its structure is planar with a bond angle of \[{{120}^{o}}\].

(5) Reactions involving carbocations
(i) Electrophilic addition reactions
(ii) \[S{{N}^{1}}\] reactions
(iii) Electrophilic substitution reactions
(iv) Molecular rearrangement reactions, e.g., Pinacole-Pinacolone rearrangement.
(6) Rearrangement in carbocations
(i) Rearrangement takes place in primary and secondary carbocations.
\[p\]-carbocation![]() ter-carbocation or sec. carbocation
ter-carbocation or sec. carbocation
Driving force for rearrangement is the stability factor (Less stable species wants to convert into more stable species).
(ii) For rearrangement, carbocation should have at least three carbons. (\[\alpha ,\,\beta \] and\[\gamma \]).
(iii) If \[\beta \]-carbon has hydrogen as well as alkyl group then rearrangement is due to the migration of hydrogen in the form of hydride ion from \[\beta \] to \[\alpha \]-carbon. If \[\beta \]-carbon has only hydrogens then hydrogen migrates. If \[\beta \]-carbon has only alkyl groups then alkyl group migrates from \[\beta \] to \[\alpha \]-carbon.

![]()
· Note: If the number of products of the reaction is more than the expected products then product formation takes place by formation of carbocation and there is rearrangement in carbocation.
\[C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|\,\,\,\,\,\,\,}{\mathop{\overset{C{{H}_{3}}}{\mathop{\overset{|\,\,\,\,\,\,\,}{\mathop{C\,\,\,-}}\,}}\,}}\,}}\,C{{H}_{2}}-Cl\xrightarrow{NaOH}C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|\,\,\,\,\,\,\,}{\mathop{\overset{C{{H}_{3}}}{\mathop{\overset{|\,\,\,\,\,\,\,}{\mathop{C\,\,\,-}}\,}}\,}}\,}}\,C{{H}_{2}}OH+\underset{\text{Rearranged}\,\text{product}}{\mathop{C{{H}_{3}}-\underset{C{{H}_{3}}}{\mathop{\underset{|\,\,\,\,\,\,\,}{\mathop{\overset{OH\,\,}{\mathop{\overset{|\,\,\,\,\,\,\,}{\mathop{C\,\,\,-}}\,}}\,}}\,}}\,C{{H}_{2}}-C{{H}_{3}}}}\,\]
Carbanions
A carbanion may be defined as a species containing a carbon atom carrying a negative charge. These are generated by the heterolytic fission of covalent bond involving carbon atom in which the atom linked to carbon goes without the bonding electrons.
It is denoted by putting a negative charge (?) against the symbol of group of atoms.
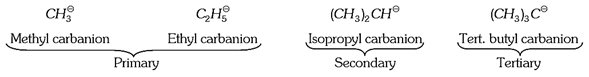
(1) Characteristics of carbanions,
(i) The formation of carbanions can be done,
(a) By attack of base on hydrogen atom: Organic compounds which possess a labile or acidic hydrogen have the tendency to produce carbanions as in the case of reactive methylene compounds which lose proton in presence of sodium ethoxide \[({{C}_{2}}{{H}_{5}}ONa)\].

(b) By decarboxylation of carboxylate ion: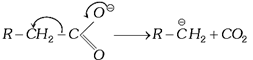
(c) By the formation of organometallic compound;
\[C{{H}_{3}}-Cl\xrightarrow{Mg/ether}C{{H}_{3}}MgCl\xrightarrow{{}}C{{\overset{}{\mathop{H}}\,}_{3}}+M{{g}^{\oplus }}C{{l}^{}}\]
(ii) Carbanions are not electrically neutral.
(iii) There are eight electrons in the outermost orbit of carbanionic carbon hence its octet is complete.
(iv) A carbanion is diamagnetic in nature due to all paired electrons.
(v) Carbanions are charged nucleophiles i.e., they react with electrophilic reagents. The order of reactivity of carbanions is, Tertiary \[({{3}^{o}})\] > Secondary \[({{2}^{o}})\] >Primary \[({{1}^{o}})\] (Decreasing order)
(2) Stability of carbanions: The stability of carbanion may be explained by
(i) Electronegativity of carbanionic carbon
Stability \[\propto \] Electronegativity of carbanionic carbon \[\propto \]% \[s\]-character of carbanionic carbon

![]()
(ii) Inductive effect: Stability of carbanions depends on the +I or ? I group as follows:
(a) Stability \[\propto \frac{1}{+I\,\text{power of the group}}\]

(b) Stability of carbanions \[\propto \,-I\] power of the group.
For example: 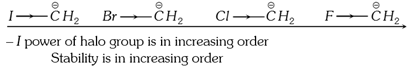
(iii) Delocalisation or Resonance: Allyl and benzyl carbanions are stabilised by delocalisation of negative charge.
\[\underset{2}{\mathop{C{{H}_{2}}=CH-\overset{}{\mathop{C}}\,{{H}_{2}}}}\,\] \[\underset{\,\,\,\,4}{\mathop{{{C}_{6}}{{H}_{5}}-}}\,\overset{}{\mathop{C}}\,{{H}_{2}}\] \[\underset{7}{\mathop{{{({{C}_{6}}{{H}_{5}})}_{2}}\overset{}{\mathop{C}}\,H}}\,\] \[\underset{10}{\mathop{{{({{C}_{6}}{{H}_{5}})}_{3}}\overset{}{\mathop{C}}\,}}\,\]
![]() Number of resonating structures is in increasing order
Number of resonating structures is in increasing order
Stability is in increasing order.
(iv) Stabilisation by sulphur and phosphorous: Attachment of carbanionic carbon of a sulphur and phosphorus atom causes an increase in carbanion stability.
The cause of stability is due to the delocalisation of negative charge of carbanion by vaccant
![]()
-orbital x\[p\pi -d\pi \] bonding) of phosphorus and sulphur.
(v) Stabilisation by >C=O, ?NO2 and CN groups present on carbanionic carbon: These groups stabilise carbanion by resonance effect.
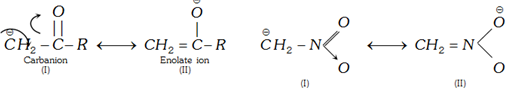
Contribution of structure (II) will be more than (I) because in (II) negative charge is present on electronegative oxygen.
(vi) Stability of Aromatic carbanions
(a) Anions in which negative charge is present on carbon of aromatic system is known as aromatic carbanions.
(b) Aromatic carbanions are most stable carbanions.
(c) Anions obeying Huckel rule are stable because they are aromatic and there is complete delocalisation of negative charge.

(3) Relative stability of different types of carbanions in decreasing order
Aromatic carbanion > Benzyl carbanion > Allyl carbanion > \[CH\equiv \overset{}{\mathop{C}}\,\,\,\,>\]
\[C{{H}_{2}}=\overset{}{\mathop{C}}\,H\,\,>\] Alkyl carbanion 
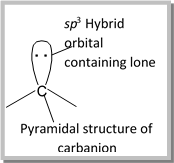
(4) Structure of carbanion: The negatively charged carbon atom in carbanion is \[s{{p}^{3}}\] hybridised. Therefore, it has a pyramidal structure. Three of the four \[s{{p}^{3}}\] hybridised orbitals form \[3\sigma \] bonds with hydrogen or carbon atom of the alkyl group. The fourth \[s{{p}^{3}}\] hybrid orbital contains the lone pair of electrons.
· Note: Geometry of allyl and benzyl carbanion is almost planar and hybridisation is\[s{{p}^{2}}\].
· Methyl anion is isostructural (pyramidal) and isoelectronic (eight valency electrons) with ammonia.
· The carbanions having three different bulky groups are not optically active. It is due to pyramidal structure of carbanion which is not rigid and shows a rapid equilibrium between following two enantiomeric pyramidal structures.
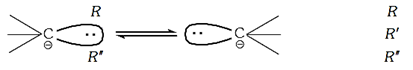
(5) Reactions involving carbanions
(i) Condensation reactions of carbonyl compounds, i.e., Aldol condensation, Perkin reaction, Reformatsky reaction etc.
(ii) Condensation reaction of ester; Claisen condensation.
(iii) Witting reaction.
Carbenes
A carbene may be defined as a species in which carbon atom has six electrons in the outer shell (electron deficient), out of which two constitute a lone pair and two are shared. So they are divalent carbon species containing two unpaired electrons and possess no charge.
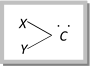
The simplest carbene is \[\overset{.\,\,.}{\mathop{C}}\,{{H}_{2}}\] which known as methylene. Substituted carbenes are simply named as derivative of carbenes. For example, \[\underset{\text{Phenyl carbene}}{\mathop{{{C}_{6}}{{H}_{5}}-\overset{.\,\,.}{\mathop{C}}\,H}}\,\] \[\underset{\text{Dialkyl carbene}}{\mathop{R-\overset{.\,\,\,.}{\mathop{C}}\,-R}}\,\] \[\underset{\text{Dichloro carbene}}{\mathop{\overset{.\,\,.}{\mathop{C}}\,C{{l}_{2}}}}\,\]
(1) Characteristics of carbnes
(i) Carbenes can be formed,
(a) By the photochemical decomposition or pyrolysis of aliphatic diazo compounds or ketenes.
\[\underset{\text{Diazo methane}}{\mathop{C{{H}_{2}}{{N}_{2}}}}\,\underset{(UV)}{\mathop{\xrightarrow{h\nu \,\text{or}\,\text{heat}}}}\,:\,\underset{\text{Carbene}}{\mathop{C{{H}_{2}}}}\,+{{N}_{2}}\]; \[R-CH{{N}_{2}}\xrightarrow{h\nu \,\text{or}\,\text{heat}}\,\underset{\text{Alkyl carbene}}{\mathop{RCH:}}\,+{{N}_{2}}\]
\[RCO-CH{{N}_{2}}\xrightarrow{h\nu \,\text{or heat}}\underset{\text{Acyl carbene}}{\mathop{RCO-CH:}}\,+{{N}_{2}}\]; \[\underset{\text{Ketene}}{\mathop{C{{H}_{2}}=C=O}}\,\xrightarrow{{}}\underset{\text{Carbene}}{\mathop{:C{{H}_{2}}}}\,+CO\]
(b) By the action of a base on suitable polyhalogen compound.
\[\underset{\text{Chloroform}}{\mathop{CHC{{l}_{3}}}}\,+{{C}_{2}}{{H}_{5}}ONa\xrightarrow{{}}\underset{\text{Dichloro carbene}}{\mathop{:CC{{l}_{2}}+}}\,{{C}_{2}}{{H}_{5}}OH+NaCl\]
\[CHC{{l}_{3}}\underset{\text{Tert}\text{. butoxide ion}}{\mathop{+{{(C{{H}_{3}})}_{3}}C{{O}^{}}}}\,\] ? \[{{(C{{H}_{3}})}_{3}}C-OH+:CC{{l}_{3}}\xrightarrow{{}}:CC{{l}_{2}}+C{{l}^{-}}\]
(ii) Carbenes are related to carbanions through the \[\alpha \]-elimination reaction.
![]() \[CH{{X}_{3}}\xrightarrow{alc.\,KOH/\Delta }\] \[{{H}^{\oplus }}+\underset{\underset{C{{X}_{2}}+{{X}^{}}}{\mathop{\overset{{}}{\mathop{.\,\,.\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}\,}}\,}{\mathop{\overset{}{\mathop{C}}\,{{X}_{3}}\,\,\,\,\,\,\,\,\,\,\,}}\,\]
\[CH{{X}_{3}}\xrightarrow{alc.\,KOH/\Delta }\] \[{{H}^{\oplus }}+\underset{\underset{C{{X}_{2}}+{{X}^{}}}{\mathop{\overset{{}}{\mathop{.\,\,.\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}}\,}}\,}{\mathop{\overset{}{\mathop{C}}\,{{X}_{3}}\,\,\,\,\,\,\,\,\,\,\,}}\,\]
(iii) Carbenes are neutral, divalent, highly reactive carbon intermediates. They works as electrophiles.
(2) Types and stability of carbenes
Carbenes are of two types
(i) Singlet: When both the electrons go into one orbital and have opposite spin (antiparallel), i.e., the unshared electrons are paired in one orbital and has a bent strucutre.

The singlet state is actually found to have \[H-C-H\] bond angle \[{{103}^{o}}\] (between \[{{90}^{o}}\] to \[{{120}^{o}}\]) and a \[C-H\] bond length of 1.12Å. It is generally considered as the less stable.
(ii) Triplet: When the two electrons go into different orbitals and have same spin (parallel), i.e., the unshared electrons are not paired. Such carbene would have a permanent magnetic moment and would exist in three closely grouped energy state, if it were placed in a magnetic field. Such a state is called triplet state and is believed to be a linear molecule.

With \[H-C-H\] bond angle \[{{180}^{o}}\] and a \[C-H\] bond length of 1.03Å, it might be considered as a free diradical and is more stable.
(3) Reactions involving carbenes
(i) Addition to alkenes: Formation of cyclo alkane derivatives, e.g.,
![]() (a)
(a) 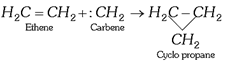
(b) 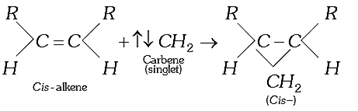
(c) 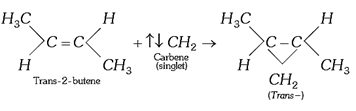
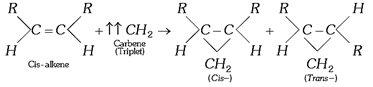
(d) Conversely, the triplet carbenes add on alkenes in a non-stereo specific manner to form both (cis- and trans-) products.
(e) In case of conjugated or cumulative dienes, the addition always occurs exclusively at 1,2-position.
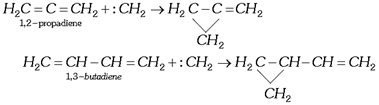
(ii) Insertion reactions: Carbenes are also used in its insertion between the \[C-H\] bond, e.g.,
\[\underset{|}{\mathop{\overset{|}{\mathop{C}}\,}}\,H+:C{{H}_{2}}\to \underset{|}{\mathop{\overset{|}{\mathop{C}}\,}}\,C{{H}_{2}}-H\] (Next homologue)
\[\underset{Methane}{\mathop{H\begin{matrix}
H \\
\underset{|}{\mathop{\overset{|}{\mathop{C}}\,}}\, \\
H \\
\end{matrix}H}}\,+:C{{H}_{2}}\to \underset{Ethane}{\mathop{H\begin{matrix}
H \\
\underset{|}{\mathop{\overset{|}{\mathop{C}}\,}}\, \\
H \\
\end{matrix}C{{H}_{2}}}}\,-H\]
Further it can be inserted in all the possible positions (pri-, sec-, tert-).
\[\underset{\text{Propane}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}H}}\,+:C{{H}_{2}}\to \underset{n-\text{butane}}{\mathop{C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}C{{H}_{3}}}}\,\] and \[\underset{\text{Iso}-\text{butane}}{\mathop{C{{H}_{3}}\underset{\underset{C{{H}_{3}}\,\,\,\,\,\,\,}{\mathop{|\,\,\,\,\,\,\,\,\,\,\,\,\,}}\,}{\mathop{CHC{{H}_{3}}}}\,}}\,\]
(iii) Ring expansion reactions: These involve the addition of a halogeno carbene across a double bond followed by rearrangement.
(a) 
(b) 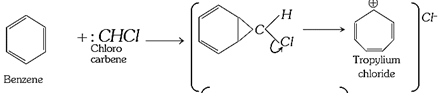
(c) 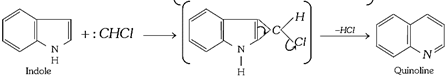
(iv) Carbylamine reaction: By heating a mixture of primary amine and chloroform with ethanolic potassium hydroxide to form isocyanide (carbylamine) is known as carbylamine reaction.
\[RN{{H}_{2}}+CHC{{l}_{3}}+3KOH\to RNC+3KCl+3{{H}_{2}}O\]
Mechanism: \[CHC{{l}_{3}}\underset{(-{{H}_{2}}O)}{\mathop{\xrightarrow{O{{H}^{-}}}}}\,\,:\overset{}{\mathop{C}}\,C{{l}_{3}}\xrightarrow{{}}\underset{\text{Dichloro carbene}}{\mathop{:CC{{l}_{2}}+}}\,C{{l}^{-}}\]
(v) Reimer-Tiemann reaction: Phenol on heating with chloroform and aqueous alkali followed by acid hydrolysis to yield salicylaldehyde is known as Reimer-Tiemann reaction.

Comparision of Free radical, Carbocation, Carbanion and Carbene
|
Characteristic |
Free radical |
Carbocation |
Carbanion |
Carbene |
|
Nature |
Neutral having odd electron |
Positive charge on C |
Negative charge on C |
Neutral, divalent with 2 unshared electrons |
|
Hybridisation |
sp2 |
sp2 |
sp3 (non-conjugated) sp2 (Conjugated) |
(i) sp2 (singlet) (ii) sp (triplet) |
|
Structure |
Planar |
Planar |
Pyramidal/Planar |
(i) Planar (singlet) (ii) Linear (triplet) |
|
Magnetism |
Paramagnetic |
Diamagnetic |
Diamagnetic |
(i) Diamagnetic (ii) Paramagnetic |
|
Stability order |
\[P{{h}_{3}}\overset{.}{\mathop{C}}\,>P{{h}_{2}}\overset{.\,\,\,\,\,\,\,}{\mathop{CH}}\,>Ph\overset{.\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,>\] \[C{{H}_{2}}=CH-\overset{.\ \ \ \ }{\mathop{C{{H}_{2}}}}\,>{{3}^{o}}>{{2}^{o}}>\]\[{{1}^{o}}>\overset{.\ \ \ \ }{\mathop{C{{H}_{2}}}}\,>C{{H}_{2}}=\overset{.\ \ \ }{\mathop{CH}}\,\] |
\[P{{h}_{3}}\overset{+}{\mathop{C}}\,>P{{h}_{2}}\overset{+\,\,\,\,\,}{\mathop{CH}}\,>\] \[\overset{+\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,}{\mathop{PhC{{H}_{2}}}}\,>C{{H}_{2}}=CH-\overset{+\,\,\,\,\,\,\,\,}{\mathop{C{{H}_{2}}}}\,>\]
\[{{3}^{o}}>{{2}^{o}}>{{1}^{o}}>C{{H}_{3}}\] |
\[\overset{\,\,\,\,\,\,\,}{\mathop{P{{h}_{3}}C}}\,>\overset{\,\,\,}{\mathop{P{{h}_{2}}CH}}\,>\] \[\overset{}{\mathop{PhC{{H}_{2}}}}\,>\text{Allyl}>\] \[\overset{}{\mathop{C{{H}_{2}}}}\,>{{1}^{o}}>{{2}^{o}}>{{3}^{o}}\] |
Triplet > singlet |
Benzyne
(1) 1, 2-Didehydrobenzene, \[{{C}_{6}}{{H}_{4}}\] and its derivatives are called benzyne or arynes and the simplest member is benzyne.
(2) It is neutral reaction intermediate derived from benzene ring by removing two substituents, of ortho positions, one in the form of electrophile and other in the from of nucleophile leaving behind two electrons to be destributed between two orbitals.
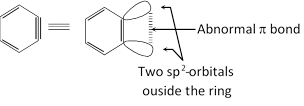
(3) Benzye intermediate is aromatic in character.
(4) When halobenzene is heated with sodamide formation of benzyne takes place.
 \[\xrightarrow{NaN{{H}_{2}}}\]
\[\xrightarrow{NaN{{H}_{2}}}\]
(5) (i) It behaves as dienophile and gives Diels-Alder reaction with diene.
(ii) It reacts with strong nucleophile like \[\overset{\,\,\,\,\,\,\,}{\mathop{N{{H}_{2}}}}\,\]
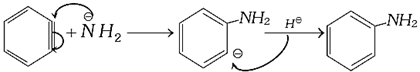
![]()
Nitrenes (R ? N :)
(1) The nitrogen analogous of carbenes are called nitrenes.
(2) There is posibility of two spin states for nitrenes depending on whether the two non-bonding electrons (the normal nitrogen lone pair remains paired) have their spins paired or parallel.

(3) In general nitrenes obey Hunds rule and are ground state triplet with two degenerate \[sp\]-orbitals containing a single electron each.

(4) Nitrenes can be generated, in situ, by the following methods,
(i) By action of \[B{{r}_{2}}\] in presence of a base on a \[{{1}^{o}}\] amide (Hofmann-bromamide reaction),
\[\underset{{{1}^{o}}\,\text{Amide}}{\mathop{R-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-N{{H}_{2}}}}\,\xrightarrow{B{{r}_{2}}/NaOH}\left[ R-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-N{{H}_{2}}\underset{-{{H}_{2}}O}{\mathop{\xrightarrow{O{{H}^{-}}}}}\,R-\overset{O}{\mathop{\overset{|\,|}{\mathop{C}}\,}}\,-\underset{.\,\,.}{\mathop{\overset{.\,\,.}{\mathop{N}}\,}}\, \right.-Br\]
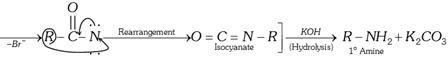
(ii) By decomposition of azides in presence of heat or light.
\[\underset{\text{Alkyl}\,\text{azide}}{\mathop{R-\overset{.\,\,.}{\mathop{N}}\,=\overset{+}{\mathop{N}}\,=\overset{.\,\,.}{\mathop{N}}\,{{:}^{-}}}}\,\,\,\xrightarrow{\Delta \,or\,\text{h }\!\!\nu\!\!\text{ }}\,\underset{\text{Alkyl}\,\text{nitrene}}{\mathop{R-\overset{.\,\,.}{\mathop{N}}\,:}}\,+N\equiv N\]
(iii) Unsubstituted nitrene \[(H-\overset{.\,\,.}{\mathop{N}}\,:)\] can be obtained by photolysis of (or by passing electric discharge through) \[N{{H}_{3}},\,{{N}_{2}}{{H}_{4}}\] or \[{{N}_{3}}H\].
You need to login to perform this action.
You will be redirected in
3 sec
