Discovery and Properties of anode, cathode rays neutron and Nuclear structure
Category : NEET
Discovery and Properties of anode, Cathode rays neutron and Nuclear structure
Discovery and Properties of anode, Cathode rays
John Dalton 1808, believed that matter is made up of extremely minute indivisible particles, called atom which can takes part in chemical reactions. These can neither be created nor be destroyed. However, modern researches have conclusively proved that atom is no longer an indivisible particle. Modern structure of atom is based on Rutherford’s scattering experiment on atoms and on the concepts of quantization of energy.
Composition of atom.
The works of J.J. Thomson and Ernst Rutherford actually laid the foundation of the modern picture of the atom. It is now believed that the atom consists of several sub-atomic particles like electron, proton, neutron, positron, neutrino, meson etc. Out of these particles, the electron, proton and the neutron are called fundamental subatomic particles and others are non-fundamental particles.
Electron (–1eo)
(1) It was discovered by J.J. Thomson (1897) and is negatively charged particle. Electron is a component particle of cathode rays.
(2) Cathode rays were discovered by William Crooke's & J.J. Thomson (1880) using a cylindrical hard glass tube fitted with two metallic electrodes. The tube has a side tube with a stop cock. This tube was known as discharge tube. They passed electricity (10,000V) through a discharge tube at very low pressure (\[{{10}^{-2}}\] to \[{{10}^{-3}}mm\,Hg)\]. Blue rays were emerged from the cathode. These rays were termed as Cathode rays.
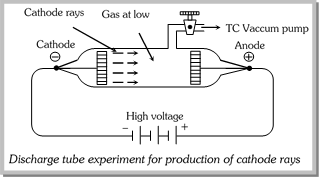
(3) Properties of Cathode rays
(i) Cathode rays travel in straight line.
(ii) Cathode rays produce mechanical effect, as they can rotate the wheel placed in their path.
(iii) Cathode rays consist of negatively charged particles known as electron.
(iv) Cathode rays travel with high speed approaching that of light (ranging between \[{{10}^{-9}}\] to \[{{10}^{-11}}\] cm/sec)
(v) Cathode rays can cause fluorescence.
(vi) Cathode rays heat the object on which they fall due to transfer of kinetic energy to the object.
(vii) When cathode rays fall on solids such as \[Cu,\,X-\]rays are produced.
(viii) Cathode rays possess ionizing power i.e., they ionize the gas through which they pass.
(ix) The cathode rays produce scintillation the photographic plates.
(x) They can penetrate through thin metallic sheets.
(xi) The nature of these rays does not depend upon the nature of gas or the cathode material used in discharge tube.
(xii) The e/m (charge to mass ratio) for cathode rays was found to be the same as that for an \[{{e}^{-}}\] \[(-1.76\times {{10}^{8}}\] coloumb per gm). Thus, the cathode rays are a stream of electrons..
![]()
Note : q When the gas pressure in the discharge tube is 1 atmosphere no electric current flows
through the tube. This is because the gases are poor conductor of electricity.
q The television picture tube is a cathode ray tube in which a picture is produced due to fluorescence on the television screen coated with suitable material. Similarly, fluorescent light tubes are also cathode rays tubes coated inside with suitable materials which produce visible light on being hit with cathode rays.
(4) R.S. Mullikan measured the charge on an electron by oil drop experiment. The charge on each electron is \[-1.602\times {{10}^{-19}}C.\]
(5) Name of electron was suggested by J.S. Stoney. The specific charge (e/m) on electron was first determined by J.J. Thomson.
(6) Rest mass of electron is\[9.1\times {{10}^{-28}}gm\]\[=0.000549amu=1/1837\] of the mass of hydrogen atom.
(7) According to Einstein’s theory of relativity, mass of electron in motion is, \[{m}'\] \[=\frac{\text{Rest mass of electron(m) }}{\sqrt{[1-{{(u/c)}^{2}}]}}\]
Where \[u\]= velocity of electron, c= velocity of light.
When u=c than mass of moving electron =¥.
(8) Molar mass of electron = Mass of electron × Avogadro number =\[5.483\times {{10}^{-4}}.\]
(9) 1.1\[\times {{10}^{27}}\] electrons =1gram.
(10) 1 mole electron = \[0.5483\] mili gram.
(11) Energy of free electron is ≈ 0. The minus sign on the electron in an orbit, represents attraction between the positively charged nucleus and negatively charged electron.
(12) Electron is universal component of matter and takes part in chemical combinations.
(13) The physical and chemical properties of an element depend upon the distribution of electrons in outer shells.
(14) The radius of electron is \[4.28\times {{10}^{-12}}cm.\]
(15) The density of the electron is = \[2.17\times {{10}^{-17}}g/mL\].
Example : 1 The momentum of electron moving with 1/3rd velocity of light is (in g cm sec–1)
(a) \[9.69\times {{10}^{-8}}\] (b) \[8.01\times {{10}^{10}}\] (c) \[9.652\times {{10}^{-18}}\] (d) None
Solution: (c) Momentum of electron, ‘p’ = \[{m}'\times u\]
Where \[{m}'\] is mass of electron in motion\[=\frac{m}{\sqrt{1-{{\left( u/c \right)}^{2}}}}\]; Also \[u=c/3\]
\ Momentum\[=\frac{9.108\times {{10}^{-28}}}{\sqrt{1-{{\left( \frac{c}{3\times c} \right)}^{2}}}}\times \frac{3\times {{10}^{10}}}{3}=\frac{9.108\times {{10}^{-28}}\times 3\times {{10}^{10}}}{0.94\times 3}=9.652\times {{10}^{-18}}\,g\,\,cm\,\,{{\sec }^{-1}}\]
Example: 2 An electron has a total energy of 2 MeV. Calculate the effective mass of the electron in kg and its speed. Assume rest mass of electron 0.511 MeV.
(a) \[2.9\times {{10}^{8}}\] (b) \[8.01\times {{10}^{8}}\] (c) \[9.652\times {{10}^{8}}\] (d) None
Solution: (a) Mass of electron in motion \[=\frac{2}{931}amu\] (1 amu = 931 MeV)
\[=\frac{2}{931}\times 1.66\times {{10}^{-27}}kg\]\[=3.56\times {{10}^{-30}}kg\] (1\[amu=1.66\times {{10}^{-27}}kg\])
Let the speed of the electron be u.
\[{m}'=\frac{m}{\sqrt{1-{{\left( u/c \right)}^{2}}}}\] or \[3.56\times {{10}^{-30}}=\frac{\frac{0.511}{931}\times 1.66\times {{10}^{-27}}}{\sqrt{1-{{\left( \frac{u}{3\times {{10}^{8}}} \right)}^{2}}}}=\frac{0.911\times {{10}^{-30}}}{\sqrt{1-{{\left( \frac{u}{3\times {{10}^{8}}} \right)}^{2}}}}\]
or \[1-{{\left( \frac{u}{3\times {{10}^{8}}} \right)}^{2}}=0.06548\] or \[{{u}^{2}}=9\times {{10}^{16}}\times 0.93452\] or \[u=2.9\times {{10}^{8}}m\]
Example: 3 A electron of rest mass \[1.67\times {{10}^{-27}}\] kg is moving with a velocity of 0.9c (c = velocity of light). Find its mass and momentum.
(a) \[10.34\times {{10}^{-19}}\] (b) \[8.01\times {{10}^{10}}\] (c) \[9.652\times {{10}^{-18}}\] (d) None
Solution: (a) Mass of a moving object can be calculated using Einsten’s theory of relativity :
\[{m}'=\frac{m}{\sqrt{1-{{\left( u/c \right)}^{2}}}}\] \[m=\] rest mass (given), u = velocity (given), c = velocity of light
\[{m}'=\frac{1.67\times {{10}^{-27}}}{\sqrt{1-{{\left( \frac{0.9c}{c} \right)}^{2}}}}=3.83\times {{10}^{-27}}kg\]
Momentum \['p'={m}'\times u\]
\[p=3.83\times {{10}^{-27}}\times 0.9c=10.34\times {{10}^{-19}}kg\,\,m{{s}^{-1}}\]
Proton (1H1, H+, P)
(1) Proton was discovered by Goldstein and is positively charged particle. It is a component particle of anode rays.
(2) Goldstein (1886) used perforated cathode in the discharge tube and repeated Thomson's experiment and observed the formation of anode rays. These rays also termed as positive or canal rays.
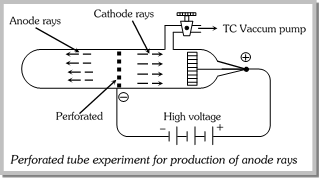
(3) Properties of anode rays
(i) Anode rays travel in straight line.
(ii) Anode rays are material particles.
(iii) Anode rays are positively charged.
(iv) Anode rays may get deflected by external magnetic field.
(v) Anode rays also affect the photographic plate.
(vi) The e/m ratio of these rays is smaller than that of electrons.
(vii) Unlike cathode rays, their e/m value is dependent upon the nature of the gas taken in the tube. It is maximum when gas present in the tube is hydrogen.
(viii) These rays produce flashes of light on ZnS screen.
(4) Charge on proton = \[1.602\times {{10}^{-19}}\]coulombs = \[4.80\times {{10}^{-10}}e.s.\,u.\]
(5) Mass of proton = Mass of hydrogen atom= \[1.00728amu\,\]\[=1.673\times {{10}^{-24}}gram\]\[=1837\] of the mass of electron.
(6) Molar mass of proton = mass of proton \[\times \]Avogadro number \[=1.008\] (approx).
(7) Proton is ionized hydrogen atom \[({{H}^{+}})\] i.e., hydrogen atom minus electron is proton.
(8) Proton is present in the nucleus of the atom and it's number is equal to the number of electron.
(9) Mass of 1 mole of protons is \[\approx \]1.007 gram.
(10) Charge on 1 mole of protons is \[\approx \] 96500 coulombs.
(11) The volume of a proton (volume = \[\frac{4}{3}\pi {{r}^{3}}\]) is \[\approx \] 1.5\[\times {{10}^{-38}}c{{m}^{3}}.\]
(12) Specific charge of a proton is \[9.58\times {{10}^{4}}\] Coulomb/gram.
Neutron (on1, N)
(1) Neutron was discovered by James Chadwick (1932) according to the following nuclear reaction,

(2) The reason for the late discovery of neutron was its neutral nature.
(3) Neutron is slightly heavier (0.18%) than proton.
(4) Mass of neutron = \[1.675\times {{10}^{-24}}gram\]= \[1.675\times {{10}^{-27}}kg\]=\[1.00899\,amu\approx \,\] mass of hydrogen atom.
(5) Specific charge of a neutron is zero.
(6) Density = \[1.5\times {{10}^{-14}}gram/c.c.\]
(7) 1 mole of neutrons is \[\approx \] 1.008 gram.
(8) Neutron is heaviest among all the fundamental particles present in an atom.
(9) Neutron is an unstable particle. It decays as follows :
\[\underset{\text{neutron}}{\mathop{_{0}{{n}^{1}}}}\,\xrightarrow{{}}\,\underset{\text{proton}}{\mathop{_{1}{{H}^{1}}}}\,\,\,+\,\underset{\text{electron}}{\mathop{_{-1}{{e}^{0}}}}\,+\underset{\text{anti nutrino}}{\mathop{{{\,}_{0}}{{\nu }^{0}}}}\,\]
(10) Neutron is fundamental particle of all the atomic nucleus, except hydrogen or protium.
Comparison of mass, charge and specific charge of electron, proton and neutron
Name of constant |
Unit |
Electron(e–) |
Proton(p+) |
Neutron(n) |
|
Mass (m) |
amu kg Relative |
0.000546 9.109 × 10–31 1/1837 |
1.00728 1.673 × 10–27 1 |
1.00899 1.675 × 10–24 1 |
|
Charge(e)
|
Coulomb (C) esu Relative |
– 1.602 × 10–19 – 4.8 × 10–10 – 1 |
+1.602 × 10–19 +4.8 × 10–10 +1 |
Zero Zero Zero |
|
Specific charge (e/m) |
C/g |
1.76 × 108 |
9.58 × 104 |
Zero |
Other non-fundamental particles
|
Particle |
Symbol |
Nature |
Charge esu ´10?10 |
Mass (amu) |
Discovered by |
|
Positron |
\[{{e}^{+}},\,1{{e}^{0}},\,{{\beta }^{+}}\] |
+ |
+ 4.8029 |
0.0005486 |
Anderson (1932) |
|
Neutrino |
n |
0 |
0 |
< 0.00002 |
Pauli (1933) and Fermi (1934) |
|
Anti-proton |
\[{{p}^{-}}\] |
? |
? 4.8029 |
1.00787 |
Chamberlain Sugri (1956) and Weighland (1955) |
|
Positive mu meson |
\[{{\mu }^{+}}\] |
+ |
+ 4.8029 |
0.1152 |
Yukawa (1935) |
|
Negative mu meson |
\[{{\mu }^{-}}\] |
? |
? 4.8029 |
0.1152 |
Anderson (1937) |
|
|
\[{{\pi }^{+}}\] |
+ |
+ 4.8029 |
0.1514 |
Powell (1947) . |
|
Negative pi meson |
|
? |
? 4.8029 |
0.1514 |
|
|
Neutral pi meson |
|
0 |
0 |
0.1454 |
You need to login to perform this action.
You will be redirected in
3 sec
