Adsorption and Adsorption isotherm
Category : NEET
Adsorption and Adsorption isotherm Adsorption. (1) Definition : The phenomenon of attracting and retaining the molecules of a substance on the surface of a liquid or solid resulting in to higher concentration of the molecules on the surface is called adsorption. (2) Causes of adsorption : Unbalanced forces of attraction or free valencies which is present at the solid or liquid surface, have the property to attract and retain the molecules of a gas or a dissolved substance on to their surfaces with which they come in contact.
Example : (i) Ammonia gas placed in contact with charcoal gets adsorbed on the charcoal whereas ammonia gas placed in contact with water gets absorbed into water, giving \[N{{H}_{4}}OH\] solution of uniform concentration. (ii) If silica gel is placed in a vessel containing water vapours, the latter are adsorbed on the former. On the other hand, if anhydrous \[CaC{{l}_{2}}\] is kept in place of silica gel, absorption takes places as the water vapours are uniformly distributed in \[CaC{{l}_{2}}\] to form hydrated calcium chloride \[(CaC{{l}_{2}}.\ 2{{H}_{2}}O)\]. Some basic terms which are used in adsorption
Interface : Any surface is a plane which separates any two phases in contact with each other. The plane which separates any two phase is generally called an interface between the two phases. 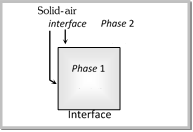 |
Adsorbate and Adsorbent: The substance which gets adsorbed on any surface is called adsorbate for example, if a gas gets adsorbed on to the surface of a solid, then the gas is termed as the adsorbate. The substance on the surface of which adsorption takes place is called adsorbent. 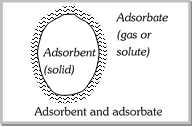 Adsorbent may be a solid or a liquid metal powders. Powdered charcoal, animal charcoal silica powder etc. are commonly used as adsorbents. Adsorbent may be a solid or a liquid metal powders. Powdered charcoal, animal charcoal silica powder etc. are commonly used as adsorbents. |
Desorption : The removal of the adsorbed substance from a surface is called desorption. 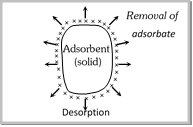 |
Absorption : When the molecules of a substance are uniformly distributed throughout the body of a solid or liquid. This phenomenon is called absorption. 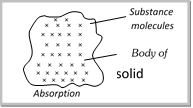 |
Sorption : The phenomenon in which adsorption and absorption occur simultaneously is called sorption. Mc. Bain introduced a general term sorption describeing both the processes, however adsorption is instantaneous i.e. a fast process while absorption is a slow process. 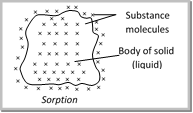 |
Occlusion : When adsorption of gases occur on the surface of metals this is called occlusion. 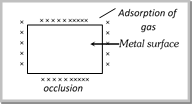 |
Difference between adsorption and absorption
| Adsorption | Absorption |
| It is a surface phenomenon. | It concerns with the whole mass of the absorbent. |
| In it, the substance is only retained on the surface and does not go into the bulk or interior of the solid or liquid. | It implies that a substance is uniformly distributed, through the body of the solid or liquid. |
| In it the concentration of the adsorbed molecules is always greater at the free phase. | In it the concentration is low. |
| It is rapid in the beginning and slows down near the equilibrium. | It occurs at the uniform rate. |
| Examples : (i) Water vapours adsorbed by silica gel. (ii) NH3 is adsorbed by charcoal. (iii) N2 is adsorbed on mica. (iv) O2 is adsorbed on tungsten surface (v) Decolourisation of sugar solution by activated or animal charcoal. (vi) Ink is adsorbed by blotting paper. | Examples : (i) Water vapours absorbed by anhydrous CaCl2 (ii) NH3 is absorbed in water forming NH4OH |
(3) Surface forces : Only the surface atoms of an adsorbent play an active role in adsorption. These atoms posses unbalanced forces of various types such as, Vander Waal?s forces and chemical bond forces.
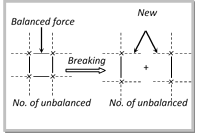
Thus, the residual force-field on a free surface which is responsible for adsorption is produced. For example, when a solid substance is broken into two pieces, two new surfaces are formed and therefore, the number of unbalanced forces becomes more. As a result the tendency for adsorption become large.
(4) Reversible and Irreversible adsorption : The adsorption is reversible, if the adsorbate can be easily removed from the surface of the adsorbent by physical methods.If the adsorbate can not be easily removed from the surface of the adsorbent is called irreversible adsorption.
(i) Example for Reversible adsorption: A gas adsorbed on a solid surface can be completely removed in vacuum.
(ii) Example for Irreversible adsorption: Adsorption of O2 on tungusten adsorbent.
(5) Characteristics of adsorption
(i) Adsorption refers to the existence of a higher concentration of any particular component at the surface of a liquid or a solid phase.
(ii) Adsorption is accompanied by decrease in the \[\Delta G\] (free energy charge) of the system when \[\Delta G=0\], adsorption equilibrium is said to be established.
(iii) Adsorption is invariably accompanied by evolution of heat, i.e. it is an exothermic process. In other words, DH of adsorption is always negative.
(iv) When a gas is adsorbed, the freedom of movement of its molecules becomes restricted. On account of it decrease in the entropy of the gas after adsorption, i.e. DS is negative.
Adsorption is thus accompanied by decrease in enthalpy as well as decrease in entropy of the system and \[\Delta G\] also decreases.
(v) For a process to be spontaneous, the thermodynamic requirement is that \[\Delta G\] must be negative, i.e. there is decrease in free energy. On the basis of Gibb?s Helmholtz equation, \[\Delta G=\Delta H-T\Delta S\] ,\[\Delta G\] can be negative if \[\Delta H\] has sufficiently high negative value and \[T\Delta S\] has positive value.
![]()
Note :q When adsorbents are porous, the adsorbate is actually condensed in the pores. This is called capillary condensation. Classification of Adsorption. Adsorption can be classified into two categories as described below.
(1) Depending upon the concentration : In adsorption the concentration of one substance is different at the surface of the other substance as compared to adjoining bulk or interior phase.
(i) Positive adsorption : If the concentration of adsorbate is more on the surface as compared to its concentration in the bulk phase then it is called positive adsorption. Example : When a concentrated solution of KCl is shaken with blood charcoal, it shows positive adsorption.
(ii) Negative adsorption : If the concentration of the adsorbate is less than its concentration in the bulk then it is called negative adsorption. Example : When a dilute solution of KCl is shaken with blood charcoal, it shows negative adsorption.
(2) Depending upon the nature of force existing between adsorbate molecule and adsorbent
(i) Physical adsorption : If the forces of attraction existing between adsorbate and adsorbent are Vander Waal?s forces, the adsorption is called physical adsorption. This type of adsorption is also known as physisorption or Vander Waal?s adsorption. It can be easily reversed by heating or decreasing the pressure.
(ii) Chemical adsorption : If the forces of attraction existing between adsorbate particles and adsorbent are almost of the same strength as chemical bonds, the adsorption is called chemical adsorption. This type of adsorption is also called as chemisorption or Langmuir adsorption. This type of adsorption cannot be easily reversed. Comparison between physisorption and chemisorption
| Physisorption (Vander Waal's adsorption) | Chemisorption (Langmuir adsorption) |
| Low heat of adsorption usually in range of 20-40 kJ/mol | High heat of adsorption in the range of 50-400 kJ/mol |
| Force of attraction are Vander Waal's forces. | Forces of attraction are chemical bond forces. |
| It is reversible | It is irreversible |
| It is usually takes place at low temperature and decreases with increasing temperature. | It takes place at high temperature. |
| It is related to the case of liquefication of the gas. | It is not related. |
| It forms multimolecular layers. | It forms monomolecular layers. |
| It does not require any activation energy. | It requires high activation energy. |
| High pressure is favourable. Decrease of pressure causes desorption | High pressure is favourable. Decrease of pressure does not cause desorption. |
| It is not very specific. | It is highly specific. |
![]()
:q Adsorption of gases on animal charcoal and adsorption of water vapours on silica gel is physical adsorption.
q The behavior of adsorption of N2 on iron clearly distinguishes between physisorption and chemisorption. At 83 K, nitrogen is physisorbed on iron surface as N2 molecules. The amount of N2 adsorbed decreases rapidly as the temperature increases at room temperature, practically, there is no adsorption of N2 on iron. However at 773 K and above, nitrogen is chemisorbed on the iron surface as nitrogen atoms.
q Due to formation of multilayers physical adsorption decreases after some times.
q Chemisorption and physisorption both are exothermic.
Adsorption of gases on solids.
Factors which affect the extent of adsorption on solid surface : The following are the factors which affect the adsorption of gases on solid surface.
(1) Nature of the adsorbate (gas) and adsorbent (solid)
(i) In general, easily liquefiable gases e.g., CO2, NH3, Cl2 and SO2 etc. are adsorbed to a greater extent than the elemental gases e.g. H2, O2, N2, He etc. (while chemisorption is specific in nature.)
(ii) Porous and finely powdered solid e.g. charcoal, fullers earth, adsorb more as compared to the hard non-porous materials. Due to this property powdered charcoal is used in gas masks.
(2) Surface area of the solid adsorbent
(i) The extent of adsorption depends directly upon the surface area of the adsorbent, i.e. larger the surface area of the adsorbent, greater is the extent of adsorption.
(ii) Surface area of a powdered solid adsorbent depends upon its particle size. Smaller the particle size, greater is its surface area.
(iii) The surface area per gram of the adsorbent is called specific surface area of the adsorbent.
(3) Effect of pressure on the adsorbate gas
(i) An increase in the pressure of the adsorbate gas increases the extent of adsorption.
(ii) At low temperature, the extent of adsorption increases rapidly with pressure.
(iii) Small range of pressure, the extent of adsorption is found to be directly proportional to the pressure.
(iv) At high pressure (closer to the saturation vapour pressure of the gas), the adsorption tends to achieve a limiting value. 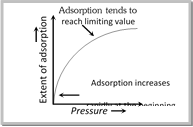
(4) Effect of temperature
(i) As adsorption is accompanied by evolution of heat, so according to the Le-Chatelier’s principle, the magnitude of adsorption should decrease with rise in temperature.
(ii) The relationship between the extent of adsorption and temperature at any constant pressure is called adsorption isobar.
(iii) A physical adsorption isobar shows a decrease in x/m (where ‘m’ is the mass of the adsorbent and ‘x’ that of adsorbate) as the temperature rises.
(iv) The isobar of chemisorption show an increase in the beginning and then decrease as the temperature rises.
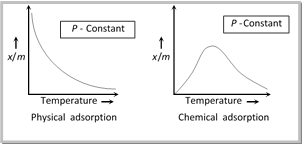
(5) Activation of adsorbent
(i) Activation of an adsorbent means, increase in the adsorbing power of the adsorbent.
(ii) This can be achieved by increasing the surface area of the adsorbent.
(iii) This can be done by making the surface of adsorbent rough or by breaking it into small pieces.
(iv) If the particle are made of then interparticle space will be too small hence the extent of adsorption may decrease.
(v) The active sites (clear surface) can be actually free from the adsorbed gases by heating in very high vacuum (\[{{10}^{-10}}\]or \[{{10}^{-11}}\]mm Hg)
Adsorption isotherms.
\[\text{Extent}\,\text{of}\,\text{adsorption}\,=\frac{x}{m}\]
Various adsorption isotherms are commonly employed in describing the adsorption data.
(1) Freundlich adsorption isotherm
(i) Freundlich adsorption isotherm is obeyed by the adsorptions where the adsorbate forms a monomolecular layer on the surface of the adsorbent.
\[\frac{x}{m}=k{{p}^{\frac{1}{n}}}\] (Freundlich adsorption isotherm) or \[\log \frac{x}{m}=\log k+\frac{1}{n}\log p\] where, x is the weight of the gas adsorbed by m gm of the adsorbent at a pressure p, thus x/m represents the amount of gas adsorbed by the adsorbents per gm (unit mass), k and n are constant at a particular temperature and for a particular adsorbent and adsorbate (gas), n is always greater than one, indicating that the amount of the gas adsorbed does not increase as rapidly as the pressure.
(ii) At low pressure, the extent of adsorption varies linearly with pressure. \[\frac{x}{m}\propto p'\]
(iii) At high pressure, it becomes independent of pressure. \[\frac{x}{m}\propto {{p}^{0}}\]
(iv) At moderate pressure \[\frac{x}{m}\] depends upon pressure raised to powers \[\frac{x}{m}\propto {{p}^{\frac{1}{n}}}\]
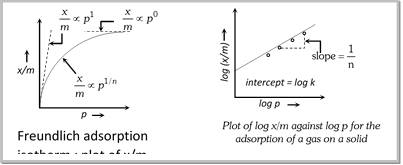
![]()
:q Equation \[\log \frac{x}{m}=\log k+\frac{1}{n}\log p\]is similar to the equation of a straight line \[y=c+mx\] . Therefore, the plot of log (x/m) against log p should be a straight line with an intercept equal to log k and slope \[\frac{1}{n}\].
(2) The Langmuir - adsorption isotherms
(i) One of the drawbacks of Freundlich adsorption isotherm is that it fails at high pressure of the gas. Irving Langmuir in 1916 derived a simple adsorption isotherm, on theoretical considerations based on kinetic theory of gases. This is named as Langmuir adsorption isotherm.
(ii) The main points of Langmuir’s theory of adsorption are as follows,
(a) Adsorption takes place on the surface of the solid only till the whole of the surface is completely covered with a unimolecular layer of the adsorbed gas.
(b) Adsorption consists of two opposing processes, namely Condensation of the gas molecules on the solid surface and Evaporation (desorption) of the gas molecules from the surface back into the gaseous phase. (c) The rate of condensation depends upon the uncovered (bare) surface of the adsorbent available for condensation. Naturally, at start when whole of the surface is uncovered the rate of condensation is very high and as the surface is covered more and more, the rate of condensation progressively decreases. On the contrary, the rate of evaporation depends upon the covered surface and hence increases as more and more of the surface is covered ultimately an equilibrium will be set up at a stage when the rate of condensation becomes equal to the rate of evaporation (adsorption equilibrium).
(d) The rate of condensation also depends upon the pressure of the gas since according the kinetic theory of gases, the number of molecules striking per unit area is proportional to the pressure.
Mathematically, \[\frac{x}{m}=\frac{ap}{1+bp}\], where a and b are constants and their value depends upon the nature of gas (adsorbate), nature of the solid adsorbent and the temperature. Their values can be determined from the experimental data.
(iii) Limitation of Langmuir theory
(a) Langmuir’s theory of unimolecular adsorption is valid only at low pressures and high temperatures.
(b) When the pressure is increased or temperature is lowered, additional layers are formed. This has led to the modern concept of multilayer adsorption.
![]()
:q The Langmuir adsorption isotherm is restricted to the formation of unimolecular layer of gas molecules on the surface of solids. However, it was suggested that there is possibility of multimolecular layer of gas molecules on the surface of the solids rather than single layer. On this basis, Brunauer, Emmett and Teller proposed a new theory known as B.E.T theory.
Adsorption from Solutions.
(1) The process of adsorption can take place from solutions also.
(2) In any solution, there are two (or more) components ; solute and solvent. The solute may be present in the molecular or ionic form.
(3) The extent of adsorption from solution depends upon the concentration of the solute in the solution, and can be expressed by the Freundlich isotherm.
(4) The Freundlich adsorption isotherm for the adsorption from solution is, \[\frac{x}{m}=k{{c}^{\frac{1}{n}}}\] where, x is the mass of the solute adsorbed, m is the mass of the solid adsorbent, c is the equilibrium concentration of the solute in the solution, n is a constant having value greater than one,
k is the proportionality constant, (The value of k depends upon the nature of solid, its particle size, temperature, and the nature of solute and solvent etc.)
(5) The plot of x/m against c is similar to that Freundlich adsorption isotherm. The above equations may be written in the following form, \[\log \frac{x}{m}=\log k+\frac{1}{n}\log c\] where c, is the equilibrium concentration of the solute in the solution.
![]()
Adsorption Isostere : Degree of adsorption depends on temperature as well as on pressure. When temperature increases the extent of adsorption decreases. A linear relationship should exist between temperature and pressure with a certain amount of adsorption. The plot of temperature versus pressure for a given amount of adsorption is called adsorption isostere.
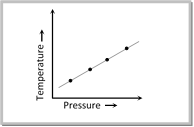
Application of Adsorption.
The phenomenon of adsorption finds a number of applications. Important applications are given as follows.
(1) Production of high vacuum : A bulk of charcoal cooled in liquid air is connected to a vessel which has already been exhausted as for as possible by a vacuum pump. The remaining traces of air are adsorbed by the charcoal. Then a very high vacuum is produced.
(2) In Gas masks : It is a device which consists of activated charcoal or a mixture of adsorbents. This apparatus is used to adsorb poisonous gases (e.g. \[C{{l}_{2}},\,CO,\,\]oxide of sulphur etc.) and thus purify the air for breathing.
(3) For desiccation or dehumidification: Certain substances have a strong tendency to absorb water such as silica and alumina \[(A{{l}_{2}}{{O}_{3}})\]. These substances can be used to reduce/remove water vapours or moisture present in the air. Silica gel is also used for dehumidification in electronic equipment.
(4) Removel of colouring matter from solution : (i) Animal charcoal removes colours of solutions by adsorbing coloured impurities. (ii) Animal charcoal is used as decolouriser in the manufacture of cane sugar.
(5) Heterogeneous catalysis : Mostly heterogeneous catalytic reactions proceed through the adsorption of gaseous reactants on solid catalyst. For example,
(i) Finely powdered nickel is used for the hydrogenation of oils.
(ii) Finely divided vanadium pentaoxide \[({{V}_{2}}{{O}_{5}})\] is used in the contact process for the manufacture of sulphuric acid.
(iii) Pt, Pd are used in many industrial processes as catalyst.
(iv) Manufacture of ammonia using iron as a catalyst.
(6) Separation of inert gases : Due to the difference in degree of adsorption of gases by charcoal, a mixture of inert gases can be separated by adsorption on coconut charcoal at different low temperatures.
(7) Softening of hard water
(i) The hard water is made to pass through a column packed with zeolite (sodium aluminium silicate) (ii) Ca++, Mg++ ions which are responsible for hardness, get adsorbed on zeolite, exchanging sodium ions.
\[N{{a}_{2}}A{{l}_{2}}S{{i}_{2}}{{O}_{8}}+CaC{{l}_{2}}\xrightarrow{{}}CaA{{l}_{2}}S{{i}_{2}}{{O}_{8}}+2NaCl\]
(iii) The exhausted zeolite is regenerated with 10% of sodium chloride solution.
\[CaA{{l}_{2}}S{{i}_{2}}{{O}_{8}}+2NaCl\xrightarrow{{}}N{{a}_{2}}A{{l}_{2}}S{{i}_{2}}{{O}_{8}}+CaC{{l}_{2}}\]
(8) De-ionisation of water
(i) Water can be de-ionised by removing all dissolved salts with the help of cation and anion-exchanger resin.
(ii) Cation-exchanger is an organic synthetic resin such as polystyrene-containing a macroanion \[(R-SO_{3}^{-}\,\text{etc}.)\]which has adsorbed H+ ions.
(iii) A resin containing a basic group \[({{R}_{3}}N_{{}}^{+}\,\text{etc}\text{.})\] which has adsorbed \[O{{H}^{-}}\] ions acts as anion exchanger.
(9) In curing diseases : A number of drugs are adsorbed on the germs and kill them or these are adsorbed on the tissues and heat them.
(10) Cleaning agents : Soap and detergents get adsorbed on the interface and thus reduce the surface tension between dirt and cloth, subsequently the dirt is removed from the cloth.
(11) Froth floatation process
(i) A low grade sulphide ore is concentrated by separating it from silica and other earthy matter by this method.
(ii) The finely divided ore is added to water containing pine oil and foaming agent.
(iii) The air is bubbled through the mixture.
(iv) The foam formed rises to the surface on which mineral particles wetted with oil are adsorbed while earthy matter settle down at the bottom.
(12) In adsorption indicators
(i) Surface of certain precipitates such as silver halide, have the property of adsorbing some dyes like eosin, fluorescein etc.
(ii) In this case of precipitation titrations (for example \[AgN{{O}_{3}}\,\,\]Versus NaCl) the indicator is adsorbed at the end point producing a characteristic colour on the precipitate.
(13) Chromatographic analysis
(i) The phenomenon of adsorption has given an excellent technique of analysis known as chromatographic analysis.
(ii) The technique finds a number of applications in analytical and industrial fields.
(iii) Chromatographic technique based on differential adsorption of different constituents of a mixture.
(14) In dyeing : Many dyes get adsorbed on the cloth either directly or by the use of mordants.
You need to login to perform this action.
You will be redirected in
3 sec
