Heat, Internal Energy and Work in Thermodynamics
Category : JEE Main & Advanced
(1) Heat \[\mathbf{(\Delta Q)}\]: It is the energy that is transferred between a system and its environment because of the temperature difference between them.
(i) Heat is a path dependent quantity e.g. Heat required to change the temperature of a given gas at a constant pressure is different from that required to change the temperature of same gas through same amount at constant volume.
(ii) For gases when heat is absorbed and temperature changes \[\Rightarrow \]\[\Delta Q=\mu C\Delta T\]
At constant pressure \[{{(\Delta Q)}_{P}}=\mu {{C}_{P}}\Delta T\]
At constant volume \[{{(\Delta Q)}_{V}}=\mu {{C}_{V}}\Delta T\]
(2) Internal energy (U) : Internal energy of a system is the energy possessed by the system due to molecular motion and molecular configuration.
The energy due to molecular motion is called internal kinetic energy \[{{U}_{K}}\] and that due to molecular configuration is called internal potential energy \[{{U}_{P}}\] i.e. Total internal energy \[U={{U}_{K}}+{{U}_{P}}\]
(i) For an ideal gas, as there is no molecular attraction \[{{U}_{p}}=0\]
i.e. internal energy of an ideal gas is totally kinetic and is given by
\[U={{U}_{K}}=\frac{3}{2}\mu RT\] and change in internal energy \[\Delta U=\frac{3}{2}\mu R\,\Delta T\]
(ii) In case of gases whatever be the process
\[\Delta U=\mu \frac{f}{2}R\Delta T\]\[=\mu {{C}_{V}}\Delta T\]\[=\mu \frac{R}{(\gamma -1)}\Delta T=\frac{\mu \,R({{T}_{f}}-{{T}_{i}})}{\gamma -1}\]
\[=\frac{\mu R{{T}_{f}}-\mu R{{T}_{i}}}{\gamma -1}\]\[=\frac{({{P}_{f}}{{V}_{f}}-{{P}_{i}}{{V}_{i}})}{\gamma -1}\]
(iii) Change in internal energy does not depend on the path of the process. So it is called a point function i.e. it depends only on the initial and final states of the system, i.e. \[\Delta U={{U}_{f}}-{{U}_{i}}\] (3) Work \[\mathbf{(\Delta W)}\]: Suppose a gas is confined in a cylinder that has a movable piston at one end. If P be the pressure of the gas in the cylinder, then force exerted by the gas on the piston of the cylinder F = PA (A = Area of cross-section of piston)
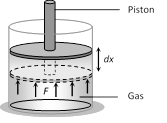
When the piston is pushed outward an infinitesimal distance dx, the work done by the gas \[dW=F.dx=P(A\,dx)=P\,dV\]
For a finite change in volume from \[{{V}_{i}}\] to \[{{V}_{f}}\]
Total amount of work done \[W=\int_{\,Vi}^{\,{{V}_{f}}}{P\,dV}=P({{V}_{f}}-{{V}_{i}})\]
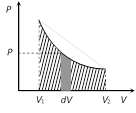
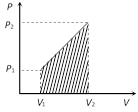
(i) If we draw indicator diagram, the area bounded by PV-graph and volume axis represents the work done
Work = Area \[=P({{V}_{2}}{{V}_{1}})\]
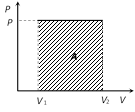
Work \[=\int_{\,{{V}_{1}}}^{{{V}_{2}}}{PdV}=P({{V}_{2}}-{{V}_{1}})\]
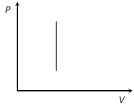
Work = 0
Work = Area of the shown trapezium \[=\frac{1}{2}({{P}_{1}}+{{P}_{2}})\,({{V}_{2}}-{{V}_{1}})\]
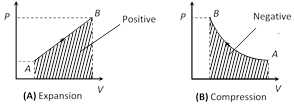
(ii) From \[\Delta W=P\Delta V=P({{V}_{f}}-{{V}_{i}})\]
If system expands against some external force then
\[{{V}_{f}}>{{V}_{i}}\] \[\Rightarrow \] \[\Delta W=\] positive
If system contracts because of external force then \[{{V}_{f}}<{{V}_{i}}\]
\[\Rightarrow \] \[\Delta W=\] negative
(iii) Like heat, work done is also depends upon initial and final state of the system and path adopted for the process
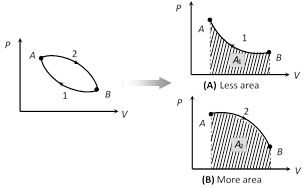
\[\because \] \[{{A}_{1}}<{{A}_{2}}\Rightarrow {{W}_{1}}<{{W}_{2}}\]
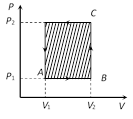
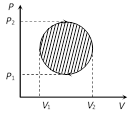
(iv) In cyclic process, work done is equal to the area of closed curve. It is positive if the cycle is clockwise and it is negative if the cycle is anticlockwise.
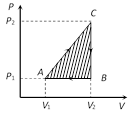
Work = Area of triangle ABC
\[=\frac{1}{2}\times ({{V}_{2}}-{{V}_{1}})\times ({{P}_{2}}-{{P}_{1}})\]
Work = Area of rectangle ABCD
\[=AB\times AD\]
\[=({{V}_{2}}{{V}_{1}})\,\,\,\,({{P}_{2}}{{P}_{1}})\]
Work = \[\frac{\pi }{4}({{P}_{2}}-{{P}_{1}})\,({{V}_{2}}-{{V}_{1}})\]
You need to login to perform this action.
You will be redirected in
3 sec
