Thermal Expansion
Category : JEE Main & Advanced

When matter is heated without any change in it's state, it usually expands. According to atomic theory of matter, a symmetry in potential energy curve is responsible for thermal expansion. As with rise in temperature the amplitude of vibration and hence energy of atoms increases, hence the average distance between the atoms increases. So the matter as a whole expands.
(1) Thermal expansion is minimum in case of solids but maximum in case of gases because intermolecular force is maximum in solids but minimum in gases.
(2) Solids can expand in one dimension (linear expansion), two dimension (superficial expansion) and three dimension (volume expansion) while liquids and gases usually suffers change in volume only.
(3) Linear expansion : When a solid is heated and it's length increases, then the expansion is called linear expansion.
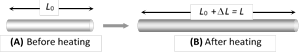
(i) Change in length \[\Delta L={{L}_{0}}\alpha \Delta T\]
(\[{{L}_{0}}=\] Original length, \[\Delta T=\] Temperature change)
(ii) Final length \[L={{L}_{0}}(1+\alpha \Delta T)\]
(iii) Co-efficient of linear expansion \[\alpha =\frac{\Delta L}{{{L}_{0}}\Delta T}\]
(iv) Unit of \[\alpha \] is \[^{o}{{C}^{-1}}\] or \[{{K}^{-1}}.\] It's dimension is \[[{{\theta }^{-1}}]\]
(4) Superficial (areal) expansion : When the temperature of a 2D object is changed, it's area changes, then the expansion is called superficial expansion.
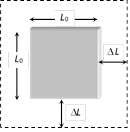
(i) Change in area is \[\Delta A={{A}_{0}}\beta \Delta T\]
(\[{{A}_{0}}=\] Original area, \[\Delta T=\] Temperature change)
(ii) Final area \[A={{A}_{0}}(1+\beta \Delta T)\]
(iii) Co-efficient of superficial expansion \[\beta =\frac{\Delta A}{{{A}_{0}}\Delta T}\]
(iv) Unit of \[\beta \] is \[^{o}{{C}^{-1}}\] or \[{{K}^{-1}}\].
(5) Volume or cubical expansion : When a solid is heated and it's volume increases, then the expansion is called volume or cubical expansion.
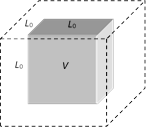
(i) Change in volume is \[\Delta V={{V}_{0}}\gamma \Delta T\]
(\[{{V}_{0}}=\] Original volume, \[\Delta T=\] change in temperature)
(ii) Final volume \[V={{V}_{0}}(1+\gamma \Delta T)\]
(iii) Volume co-efficient of expansion \[\gamma =\frac{\Delta V}{{{V}_{0}}\Delta T}\]
(iv) Unit of \[\gamma \] is \[^{o}{{C}^{-1}}\] or \[{{K}^{-1}}\].
(6) More about \[\alpha ,\,\,\beta \] and \[\gamma \] : The co-efficient \[\alpha ,\,\beta \] and \[\gamma \] for a solid are related to each other as follows
\[\alpha =\frac{\beta }{2}=\frac{\gamma }{3}\]\[\Rightarrow \] \[\alpha :\beta :\gamma =1:2:3\]
(i) Hence for the same rise in temperature
Percentage change in area \[=2\times \] percentage change in length. Percentage change in volume \[=3\times \] percentage change in length.
(ii) The three coefficients of expansion are not constant for a given solid. Their values depend on the temperature range in which they are measured.
(iii) The values of \[\alpha ,\,\,\beta ,\,\gamma \] are independent of the units of length, area and volume respectively.
(iv) For anisotropic solids \[\gamma ={{\alpha }_{x}}+{{\alpha }_{y}}+{{\alpha }_{z}}\] where \[{{\alpha }_{x}},\,\,{{\alpha }_{y}},\] and \[{{\alpha }_{z}}\] represent the mean coefficients of linear expansion along three mutually perpendicular directions.
(7) Contraction on heating : Some rubber like substances contract with rising temperature, because transverse vibration of atoms of substance dominate over longitudinal vibration which is responsible for expansion.
\[\alpha \] and \[\gamma \] for some materials
| Material | \[\alpha \,\mathbf{[}{{\mathbf{K}}^{\mathbf{-1}}}\,\mathbf{or}\,{{{{\mathbf{(}}^{\mathbf{o}}}\mathbf{C)}}^{\mathbf{-1}}}\mathbf{]}\] | \[\gamma \,\mathbf{[}{{\mathbf{K}}^{\mathbf{-1}}}\,\mathbf{or}\,{{{{\mathbf{(}}^{\mathbf{o}}}\mathbf{C)}}^{\mathbf{-1}}}\mathbf{]}\] |
| Steel | \[1.2\times {{10}^{5}}\] | \[3.6\times {{10}^{5}}\] |
| Copper | \[1.7\times {{10}^{5}}\] | \[5.1\times {{10}^{5}}\] |
| Brass | \[2.0\times {{10}^{5}}\] | \[6.0\times {{10}^{5}}\] |
| Aluminium | \[2.4\times {{10}^{5}}\] | \[7.2\times {{10}^{5}}\] |
You need to login to perform this action.
You will be redirected in
3 sec
