General Organic Chemistry
Category : Railways
General Organic Chemistry

The existence of two or more chemical compounds with the same molecular formula but having different properties owing to different arrangement of atoms within the molecule is termed as isomerism

Prefix - word root - Suffix
Word root: A word root indicates the nature of basic carbon skeleton.
|
Functional group |
Prefix/Suffix |
Functional group |
Example |
IUPAC Name |
|
1. Halogen |
Chloro, bromo, lodo |
-Cl,-Br,-I |
|
-Bromopropane |
|
2. Alcohol |
-ol |
-OH |
|
-Ethanol |
|
3. Aldehyde |
-al |
-CHO |
\[C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}CHO\] |
-Butanal |
|
4. Ketone |
-one |
-CO |
\[C{{H}_{3}}COC{{H}_{3}}\] |
-Propanone |
|
5. Carboxylic arid |
-oic acid |
-COOH |
\[C{{H}_{3}}C{{H}_{2}}COO{{H}_{{}}}\] |
-Propanoicacid |
|
6. Amine |
Ammo |
\[-N{{H}_{2}}\] |
\[C{{H}_{3}}C{{H}_{2}}N{{H}_{2}}\] |
-Amino ethane |
|
7. Ester |
oate- |
-COOR |
\[C{{H}_{3}}COOC{{H}_{3}}\] |
-Methyl ethanoate |
|
8. Double bond |
ene |
|
\[C{{H}_{3}}-CH=C{{H}_{2}}\] |
-Propene |
|
9. Triple bond |
yne |
|
\[C{{H}_{3}}-C{{H}_{2}}-C\equiv CH\] |
-Butyne |
(i) Combustion: Carbon compound undergo combustion reaction to produce \[C{{O}_{2}}\] and \[{{H}_{2}}O\] with the evolution of heat and light \[C{{H}_{4}}+{{O}_{2}}\to C{{O}_{2}}+{{H}_{2}}O+\]heat and light
(ii) Oxidation: \[\underset{ethanol}{\mathop{C{{H}_{3}}C{{H}_{2}}OH}}\,\xrightarrow{alk.\,\,KMn{{O}_{4}}/\Delta }\underset{ethanoic\,\,acid}{\mathop{C{{H}_{3}}COOH}}\,\]
The substance which are used for oxidation are known as oxidising agent, e.g alkaline\[KMn{{O}_{4}}\], acidified\[{{K}_{2}}C{{r}_{2}}{{O}_{7}}\].
(iii) Addition reaction:
Unsaturated hydrocarbons (alkenes and alkynes undergo addition reaction in presence of catalysts e.g.
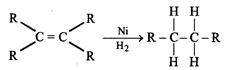
(iv) Substitution reaction: Saturated hydrocarbons give substitution reaction e.g. methane in presence of sunlight undergo chlorination.
You need to login to perform this action.
You will be redirected in
3 sec
