Elements Classification and Chemical Bonding
Category : UPSC
ELEMENTS CLASSIFICATION AND CHEMICAL BONDING
CLASSIFICATION OF ELEMENTS
Four major attempts made for classification of the elements are follows:
Dobereiner’s Triads
In 1829, J.W. Dobereiner, a German chemist made groups of three dements each and called them triads.
Newlands’ Law of Octaves
John Alexander Reina Newlands in 1863-64 noted that every eighth element showed similar physical and chemical properties, when the elements are placed in the increasing order of their atomic masses. This was called the Newlands law of octaves.
The law states that when elements are placed in the increasing order of atomic masses, the properties of the eighth elements are repeated.
Mendeleev’s Periodic Law and Periodic Table
On arranging the elements in the increasing order of atomic masses, it was observed that the elements with similar properties repeat periodically.
In 1869, Mendeleev stated his observation in the form of the following statement winch is known as the Mendeleev’s Periodic Law
The chemical and physical properties of elements are periodic function of their atomic masses.
Mendeleev arranged the elements in the form of a table which is known as the Mendeleev’s Periodic Table as below:
Salient Features of Mendeleev’s Periodic Table
The following are the main features of this periodic table:
Modern Periodic Law and Periodic Table
The Modern Periodic Law states that the chemical and physical properties of elements are periodic functions of their atomic numbers, i.e. if elements are arranged in the order of their increasing atomic number, the elements with similar properties are repeated after certain regular intervals.
The periodic table based on the modem periodic law is called the Modem Periodic Table. Presently, the accepted modem periodic table is the Long Form of Periodic Table:
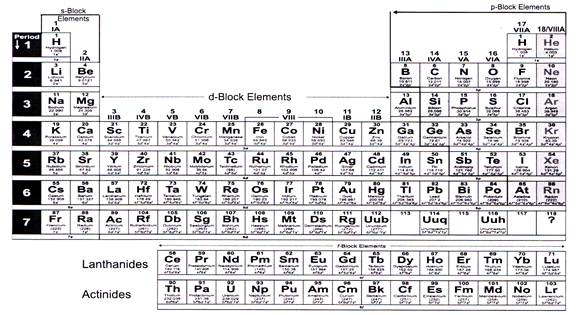
Modem Periodic table is divided into seven horizontal rows of elements. Each row of elements is called “PERIOD”. It has 18 vertical columns called groups. These groups are numbered 1 to 18 from the left, using Arabic numerals.
The electron arrangements of atoms are linked to position in the periodic table.
CLASSIFICATION OF THE ELEMENTS
The elements in the periodic table may be broadly classified as follows:
Main Group Elements
The elements present in groups 1 and 2 on left side and groups 13 to 17 on the right side of the periodic table are called representative or main group elements. Their outermost shells have less than eight electrons and hence are incomplete.
Noble Gases
Group 18 on the extreme right side of the periodic table contains noble gases. Their outermost shells contain 8 electrons except. He which contains only 2 electrons.
Their main properties are:
Transition Elements
The middle block of periodic table (groups 3 to 12) contains transition elements. Their two outermost shells are incomplete. Since these elements represent a transition (change) from the most electropositive element to the most electronegative element, they are named as transition elements.
Their important properties are as follows:
Inner Transition Elements
These elements, also called rare-earth elements, are shown separately below the main periodic table. These are two series of 14 elements each. The first series called lanthanides consists of elements having atomic number 58 to 71 (Ce to Lu). The second series of 14 rare-earth elements is called actinoids.
In all rare-earths (lanthanoids and actinoids), three outermost shells are incomplete. They are therefore called inner transition elements.
Handy Facts
It may be noted that the element lanthanum (atomic no. 57) is not a lanthanoid and the element actinium (atomic no. 89) is not an actinoid.
Metals
Metals are present in the left hand portion of the periodic table. The strong metallic elements; alkali metals (Li, Na, K, Rb, Cs, Fr) and alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra) occupy groups 1 and 2 respectively.
Non-metals
Non-metals occupy the right hand portion of the periodic table. Strong non-metallic elements i.e. halogens (F, CI, Br, I, At) and chalcogens (0, S, Se, Te, Po) occupy group 17 and 16 respectively.
Metalloids
Metalloids are located along the staircase separating the metals from the non metals on the periodic table. Boron, silicon, germanium, arsenic, antimony, and tellurium all have metal and non metal properties.
Trends in a Periodic Table (Periodic Properties)
Definite trends of certain properties of the elements are observed in the periodic table. These properties are called periodic properties. The following table depicts and describes the important periodic property and their variations:
|
Periodic property |
Definition |
Variation |
|
|
Along a period |
Down a group |
||
|
Atomic Radius |
Half of the distance between the centers of two atoms of that element that are just touching each other |
Decreases |
Increases |
|
Ionization Energy |
Energy required to completely remove an electron from a gaseous atom or ion. |
Increases |
Decreases |
|
Electron Affinity |
Energy change that occurs when an electron is added to a gaseous atom |
Increases (except for Noble Gas whose Electron Affinity is near Zero) |
|
|
Electronegativity |
Measure of the attraction of an atom for the electrons in a chemical bond. |
Increase |
Decreases |
|
Metallic Character |
Used to define the chemical properties that metallic elements present. |
Decreases |
Increases |
CHEMICAL BONDING AND REACTIONS
Chemical Bond may be defined as a force that acts between two or more atoms to hold them together as a stable molecule.
LEWIS DOT STRUCTURE
A Lewis dot symbol consists of the symbol of an element and one, dot for each valence electron in an atom of the element. The Lewis dot structure of few elements are given below:
![]()
OCTET RULE
Noble gases namely helium, neon, argon, krypton, xenon and radon do not react with other elements to form compounds i.e. they are non-reactive.
Electronic configuration of Noble gases
|
Name |
Symbol |
Atomic No. |
Electronic Configuration |
No. of electrons in the outermost cell |
|
Helium |
He |
2 |
2 |
2 |
|
Neon |
Ne |
10 |
2,8 |
8 |
|
Argon |
Ar |
18 |
2,8,8 |
8 |
|
Krypton |
Kr |
36 |
2,8,8,8 |
8 |
|
Xenon |
Xe |
54 |
2,8,18,18,8,8 |
8 |
|
Radon |
Rn |
86 |
2,8,18,32,18,8 |
8 . |
It has been concluded that
This attainment of eight electrons for stable structure is called the octet rule. The octet rule explains the chemical bonding in many compounds.
TYPES OF BONDING
Ionic Bonding
The chemical bond formed by transfer of electron from a metal to a non- metal is known as ionic or electrovalent bond.
Example:
When sodium metal and chlorine gas are brought into contact, they react violently and we obtain sodium chloride. This reaction is shown below:
\[2Na(s)+CIl2(g)\xrightarrow[{}]{{}}2NaCI(s)\]
Properties of Ionic Compounds
Ionic compounds contain ions (cations and anions) which are held together by the strong electrostatic forces of attraction. They show the following general characteristic properties:
High melting and boiling points: Ionic bonds are very strong. A. lot of energy is needed to break them. So ionic compounds have high melting and boiling points.
Conductive when liquid: Ionic compounds can only conduct electricity if their ions are free to move. Ionic compounds do not conduct electricity when they are solid. But these compounds conduct electricity when dissolved in water or melted.
Handy Facts
Ionic compounds have high melting and boiling points. The melting point of sodium chloride is 1074 K \[({{801}^{o}}C)\]and its boiling point is 1686 K\[({{1413}^{o}}C)\]. The melting and boiling points of ionic compounds are high because of he strong electrostatic forces of attraction present between the ions.
Covalent Bond
The term “covalent bond” came into use in 1939 first given by Langmuir.
A covalent bond is a chemical link between two atoms or ions where the electron pairs are shared between them. A covalent bond may also be termed as molecular bond.
Properties of covalent molecular compounds
Handy Facts
Because of the weak forces of attraction present between discrete molecules, called intermolecular forces, the covalent compounds exist as a gas or a liquid or a solid. For examples \[{{O}_{2}},{{N}_{2}},C{{O}_{2}}\]are gases; water \[CC{{I}_{4}}\]are liquids and iodine is a solid.
The melting points and boiling points of covalent compounds are lower than those of ionic compounds. For example, melting point of naphthalene which is a covalent compound is 353 K\[({{80}^{o}}C)\]. Similarly, the boiling point of carbon tetrachloride which is another covalent liquid compound is 350 K\[({{77}^{o}}C)\]
Types of covalent bonds
Polar Molecule: A molecule in which the bond dipoles present do not cancel each other out and this results in a molecular dipole,
Co-ordinate Covalent Bond
Boron reacts with the halogens to form a class of compounds having the general formula 8X3, where X is a halogen atom.
These are examples of compounds having incomplete octets.
This type of bond is called a coordinate covalent bond (also referred to as dative bond), defined as a covalent bond in which one of the atoms donates both electrons.
Exceptions to Octet Rule
The octet rule applies mainly to the second-period elements. However there are certain exceptions to the octet rules. That is there are certain compounds in which the octet rule is not satisfied. Exceptions to the octet rule fall into three categories:
An odd number of electrons
There are some molecules containing an odd number of electrons. For example, nitric oxide (NO) and nitrogen dioxide (\[N{{O}_{2}}\]). As we need an even number of electrons for complete pairing (to reach eight), the octet rule clearly cannot be satisfied for all the atoms in any of these molecules. Odd-electron molecules are sometimes called radicals.
More than eight electrons around the central atom
Atoms of the second-period elements cannot have more than eight valence electrons around the central atom, but atoms of elements in and beyond the third period of the periodic table form some compounds in which more than eight electrons surround the central atom. That is the central atom has expanded octet. One example of such a compound is\[S{{F}_{6}}\].
Metallic Bonding
The structures formed by metals are held together by metallic bond.
Handy Facts
Metals are good conductors of electricity and heat, because the free electrons carry a charge or heat energy through the metal. The free electrons allow metal atoms to slide over each other, so metals are malleable and ductile
Intermolecular Forces between Molecules
A hydrogen bond is the attractive force between the hydrogen attached to an electronegative atom of one molecule and an electronegative atom of a different molecule.
Van der Wall’s forces are forces of attraction which exist between all atoms and molecules. These forces result from weak electrostatic attraction between temporary dipoles and induced dipoles caused by movement of electrons in atoms and molecules.
CHEMICAL REACTIONS AND CHEMICAL EQUATIONS
Chemical changes result from chemical reactions taking place between different substances. It is a process in which a substance (or substances) is changed into one or more new substances.
In chemical reaction, the substance which undergoes a chemical change is called the reactant and the substance which is formed as a result of a chemical change is called the product.
Balancing Chemical Equations
According to Law of conservation of mass, the mass and the number of atoms present in the reactant(s) should be equal to the mass and number of atoms present in product(s).
The Equation can be balanced by placing the appropriate coefficient (2 in this case) in front of \[{{H}_{2}}\]and\[{{H}_{2}}O\]:
\[2{{H}_{2}}+{{O}_{2}}\xrightarrow[{}]{{}}2{{H}_{2}}O\]
This balanced chemical equation shows that “two hydrogen molecules can combine or react with one oxygen molecule to form two water molecules)”.
Handy Facts
STP in chemistry is the abbreviation for standard temperature and pressure. STP is most commonly used when performing calculation on gases, such as gas density. The standard temperature is 273 K (\[0{}^\circ \]Celsius or\[32{}^\circ \]Fahrenheit) and the standard pressure is 1 atmospheric (atm.) pressure.
Types of Chemical Reactions
The types of Chemical Reaction are discussed below with examples.
In combination reactions, as the name indicates, two or more substances (elements or compounds) simply combine to form a new substance. For example, when a substance bums it combines with oxygen present in the air.
A decomposition reaction is the one in which a compound decomposes into two or more than two substances (elements or compounds). For example, lime stone when heated gives lime and carbon dioxide.
The displacement reaction is one in which one element displaces another element from its compounds.
The reactions in which mutual exchange of radicals takes place are known as double decomposition reactions. As a result of double decomposition reactions two new substances are formed.
Oxidation and reduction reaction
When a substance gains oxygen during a reaction, it is said to be oxidized and when a substance loses oxygen during a reaction, it is said to be reduced.
Thus in this reaction, during the reaction process, one reactant gets oxidized while the other gets reduced. Such reactions are called oxidation reduction reaction or Redox Reactions.
There is no oxidation without reduction and there is no reduction without oxidation, i.e. oxidation and reduction take place simultaneously.
As per modem concept, oxidation and reduction is explained in terms of loss and gain of electrons not in terms of gain and loss of oxygen and hydrogen.
OXIDATION NUMBER
Oxidation Number shows the total number of electrons which have been removed from an element (a positive oxidation state) or added to an element (a negative oxidation state) to get to its present state.
By knowing how the oxidation number of an element changes during reaction, we can tell whether it is being oxidised or reduced.
Rules to Calculate Oxidation Numbers
Examples: Na, Mg have zero oxidation number.
Examples: \[{{H}_{2}},C{{I}_{2}},{{P}_{4}}\] have zero oxidation number.
REDOX REACTION IN DAY-TO-DAY LIFE
Redox reactions are very important in our lives. Two very common phenomena- corrosion and rancidity are results of redox reactions. These are discussed below:
Corrosion:
For most of us, Corrosion is the degradation of metals and is often called rust. Chemically we can define it as an irreversible interfacial reaction of a material (metal, ceramic, polymer) with 'is environment which results in consumption of the material. Most metals corrode on contact with water (and moisture in the air), acids, bases, salts, oils, aggressive metal polishes, and other solid and liquid chemicals. Metals will also corrode when exposed to gaseous materials like acid vapors, formaldehyde: gas, ammonia gas, and sulphur containing gases. Corrosion specifically refers to any process involving the deterioration or degradation of metal components. The best known case is that of the rusting of steel. Corrosion processes are usually electrochemical in nature.
Factors Influencing Corrosion
Following factors influence the corrosion. These are the
\[C{{O}_{2}}\], \[S{{O}_{2}}\].etc.
Methods to Prevent Corrosions:
Rancidity
What we perceive is an unpleasant change in the flavor and odour of a food, called rancidity. Unsaturated fats are more susceptible to oxidation than are saturated fats. Factors which accelerate fat oxidation include trace metals (iron, zinc, etc.), salt, light, water, bacteria, and moles. Fat oxidation can be retarded by use of antioxidants (Antioxidants are compounds that inhibit chemical reactions with oxygen such as BHT, BHA, vitamin E, and vitamin C), by use of spices such as sage and rosemary, and by use of light and/or air tight wrapping.
ENERGY CHANGES IN CHEMICAL REACTIONS
When chemical reactions take place they are often accompanied by heat changes.
You need to login to perform this action.
You will be redirected in
3 sec
