NCERT Summary - Things Around Us
Category : 6th Class
Things Around Us
All the objects are made up of one or more materials. Materials may be classified in two categories:
(1) Naturally occurring materials
(2) Man made materials
NATURALLY OCCURRING MATERIALS
· Examples are coal, wood, rocks, minerals, water, gold, petroleum, etc.
MAN MADE MATERIALS
· Examples are glass, plastic, fertiliser, paper, stainless steel, etc.
· The entire universe is made up of matter. All materials and substances are made up of matter. Anything that we can see, touch, smell or taste is matter.
· Anything that has mass and occupies space is called matter.
· Matter can exist in three different states, i.e., solid, liquid and gaseous.
(1) Solid - Example: wood
(2) Liquid - Example: water
(3) Gas - Example: oxygen
· By changing the temperature, the state of the matter can be changed. Some forms of matter can be changed from one state to another and then can be reverted to the original state.
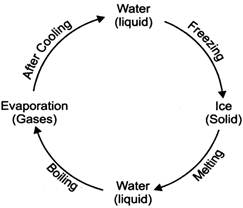
· The process of changing a solid into a liquid by heating is called melting. The melting of a substance takes place at a fixed temperature. This temperature is called the melting point of that substance.
· Ice melts at \[0{}^\circ C.\] Ice on melting forms water. On boiling water, forms steam. Steam is in a gaseous state. Most of the liquids keep on changing slowly into vapours at all temperatures. This process is called evaporation.
· On continued heating, the temperature of a liquid rises and starts boiling at a fixed temperature. This temperature is called the boiling point of the liquid. Water boils at \[100{}^\circ C.\]
· The process of changing vapour or a gas into a liquid by cooling is called condensation. For example, when water boils in a vessel, water droplets are formed due to cooling of steam on the lid and get converted into water.
· The process of changing a liquid into a solid on cooling is called freezing, as water on cooling turns into ice. \
- Gold, copper, iron and silver change into liquid and gaseous states at high temperatures.
- Materials are classified based on their state, their solubility in water, their behaviour towards a magnet, their density with respect to water, their transparency or opaqueness, etc.
- Things around us seem to be of an endless variety. Many objects and materials are made up of only a few basic units (building blocks). There are naturally occurring basic units on the Earth. These basic units are called elements. A few more elements have also been prepared by scientists. Now there are more than 110 known elements. These elements are said to be the building blocks of materials like the brick, which are the building blocks of a building.
- Some of the common elements are hydrogen, helium, carbon, nitrogen, oxygen, sodium, magnesium, aluminium, silicon, phosphorus, sulphur, zinc, bromine, silver, tin, iodine, gold and mercury. Most of the common materials are made up of one or more than one of these elements.
- Only a few elements occur in a free form in nature, for example, oxygen, nitrogen and gold. Most of the materials around us are in combinations of two or more elements,
For example:
Water: Oxygen + Hydrogen
Sugar: Carbon + Hydrogen + Oxygen
Common Salt: Sodium + Chlorine
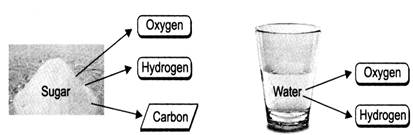
- Substances that are a combination of two or more elements are called compounds.
- The elements found in the largest quantities on Earth are silicon and oxygen. Many man-made and natural materials contain compounds of these two elements. Some examples are sand, bricks, glass, concrete, clay and granite.
- Each element has its own special properties. Therefore, elements are classified mainly in two categories, which are as follows:

METALS
Example: Iron, Copper, Silver, Gold,
Aluminium, etc.
Properties:
- Metals are good conductors of heat and electricity. That is, they allow heat and electricity to pass through them.
- Metals have lustre.
- They are generally solid.
- Mercury is the only metal, which is a liquid at normal temperatures.
NON-METALS
Example; Carbon, Sulphur,
Hydrogen, Oxygen, etc.
Properties
- Non-metals can be solids, liquids or gages.
- When two or more substances (elements or/and compounds) are mixed together in a way that they do not lose their own properties, they form a mixture.
- All elements and compounds are made of fine particles. For example, kerosene is made of fine particles of kerosene. Chalk is made of fine particles of chalk. Thus, a compound is made of fine particles of the same kind. These are the smallest particles one can see. If these particles could be broken further into even smaller particles, then one would finally get the smallest possible particle of a compound. This smallest particle cannot be seen but it still has the properties of the compound. This is called the molecule of the compound.
- Water is a commonly-used compound. It is possible to break the molecules of water. Water breaks into hydrogen and oxygen. Both have different properties. They are unlike the original substance, water.
- Hydrogen gas is made of molecules of hydrogen. Oxygen gas is made of molecules of oxygen. A molecule of hydrogen can be split further into two particles. The smallest possible particle of hydrogen is called an atom. An atom does not exist alone. Similarly, a molecule of oxygen consists of two atoms of oxygen.
- A molecule formed from the same kind of atoms is called a molecule of an element. For example, two atoms of hydrogen combine to form a molecule of the element hydrogen. A molecule which consists of atoms of two or more different elements are called molecule of a compound. For example, an atom of chlorine combines with an atom of hydrogen to form a molecule of the compound - hydrogen chloride.
- All elements are made up of only one kind of atoms. All atoms of an element are identical and alike. But the atoms of copper are different from those of aluminium. All molecules of water are identical and alike. But the molecules of sugar are different from those of water.
The molecules in all the three states of matter are similar, but they still differ in their states. The reasons are as follows:
| Reasons for differences in the three states of matter |
 |
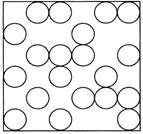
| · In solids, the molecules are closely packed. Their positions are almost fixed. |
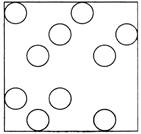
· In liquids, the molecules are not packed as closely as in solids. |
· In gases, the molecules are at a greater distance from one another as compared to solids and liquids. |
| · They have the least freedom of movement from their positions. |
· They have more freedom to move around their position in comparison to solids. |
· They are free to move around their place. |
| · Due to the above solids retain their shape. Their volume does not change. |
· Due to above reason liquids change their shape easily. |
· Due to above reason the gases, therefore, neither have a fixed shape nor fixed volume. They take the volume of the container they are kept it.. |
States of all three types of molecules

Solid Molecules Liquid Molecules Gas Molecules






