NCERT Summary - Separation of Substances
Category : 6th Class
Separation of Substances
- A mixture refers to the physical combination of two or more substances in which their individual identities are retained. The components, whether two or more, can be separated. For example, separating small pieces of stone from wheat and rice, peeling off the skin of a banana before eating it, separating cream from milk, etc.
- Separation of mixtures into their constituents is often necessary for several reasons. These are as follows:
- To remove undesirable substances
- To remove harmful substances
- To obtain a pure sample of a substance
- To obtain useful components
METHODS OF SEPARATION
- Winnowing: Winnowing is a simple method of cleaning food materials from a mixture. It separates the grains from the husk because one particle is lighter than the other. Farmers thresh wheat or paddy to loosen the grains from the chaff. This mixture is made to fall from a height. The breeze blows away the chaff, while the grains fall almost vertically. The chaff forms a separate heap at a little distance away from the heap of grains.
- Hand picking: If the mixture comprises solids of different colours, shape or sizes, it can be separated by hand picking. For example, picking stone pebbles from rice, pulses, wheat or other food grains. This method is normally used when the quantity of impurities as well as the material to be cleaned is small in quantity.
- Sieving: Sieving is possible only when the particles of a mixture are of different sizes. This method is used specially for purifying the mixtures of food materials from undesirable materials.
- Separation with magnet: Magnets attract iron. Thus, good quality magnets are used to separate iron from a mixture.
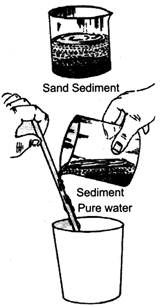
- Sedimentation and Decantation: Insoluble solids, i.e., solids that do not dissolve in a liquid can be separated from it by processes like sedimentation and decantation.
(a) Sedimentation is the process of settling down of an insoluble solid in a liquid at the bottom of a container.
(b) Decantation is the process of separating out the clear liquid on the top without disturbing the sediment. This process is only done after sedimentation.
For example, a mixture of sand and water is left for a while. The sand settles down and forms a layer at the bottom, this is sedimentation. And when clear water is poured into another vessel without disturbing the sand at the bottom, it is called decantation.
- If a solid is soluble in water or two liquids are miscible (or soluble into each other) then, the process of sedimentation cannot be used for separation. Mixture of water and sugar and mixture of kerosene oil and petrol represent a solid soluble in water and two miscible liquids, respectively.
- Two immiscible (or insoluble) liquids like a mixture of water and kerosene oil can be separated by decantation and by using a separating funnel. This method of separation is based on the property of the mixture that one constituent is heavier (water is heavier than kerosene oil) than the other. In the separating mixture, kerosene oil forms the upper layer of opening the stop-cock, water will drain off and only a small quantity of the mixture remains unseparated.
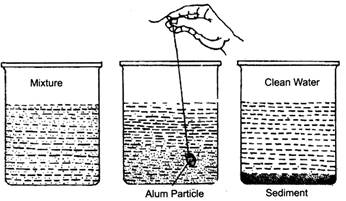
- Loading: Sometimes the insoluble particles in a liquid are very fine and cannot be removed by decantation. These particles can be made heavier so that they sediment quickly. The process of sedimentation of insoluble fine particles by making them heavier is called loading. The particles are made heavier by using alum—a solid which is soluble in water. The alum particles load the fine particles due to which they form a sediment easily and the liquid can then be cleaned.
- Centrifugation: The method of separating lighter and heavier particles from a liquid by rotating the liquid in a machine is called centrifugation. Cream is separated from milk by Centrifugation. As cream is lighter it separates and floats on the top of the milk.
- Filtration: The act of passing a liquid through a filter or a piece of equipment to remove undissolved substances is known as filtration. For example, tea leaves are separated from tea using this process.
- Evaporation: The process of converting a liquid into its vapour form is called evaporation. In this process, heat is used. The liquid containing soluble solids is heated for a sufficient time. As a result, the liquid component evaporates leaving the solid component behind. For example, salt is obtained from sea water by evaporation.
- Sublimation: It is the process of separating those substances from a mixture which convert directly into the gaseous form on heating. The gaseous form of the substance is cooled to obtain the pure solid like camphor, ammonium chloride, iodine, etc.
- Crystallisation: The process of crystallisation is used to obtain a pure sample of a soluble solid substance from a solution. For this, a large quantity of the substance is dissolved in a hot liquid. The solution is then filtered to remove insoluble impurities. On cooling, crystals of the pure solid are formed.
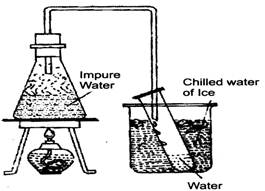
- Distillation: Distillation is a process of obtaining a pure liquid from a solution. In the process of distillation, the solution is heated to vaporise the liquid. The vapours of the liquid are cooled to get the pure liquid. This process is also used to separate two liquids that are miscible (soluble) and boil at different temperatures. For example, alcohol boils at \[80{}^\circ C\] and water boils at \[100{}^\circ C.\] If a mixture of alcohol and water is heated, alcohol boils first and distills over. The water is left behind.