Answer:
Reduction
with carbon
Electrolytic Reduction
1. Carbon is
used as a reducing agent
2. Oxides of
moderately reactive metals (e.g., Zn, Fe, Cu, Ni) are reduced by carbon.
3. In this process
the metal oxide is mixed with carbon (coke) and heated in a furnace.
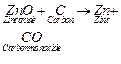
1.
Electrolysis process is used for reduction
2. Oxides
(and chlorides) of highly reactive metals (e.g., Al, Na, K, Mg, Ca) are
reduced by this process
3. In this process
molten metal oxide is electrolyzed in an electrolytic cell here the cathode
acts as a powerful reducing agent by supplying electrons to reduce metal ions
into metal.
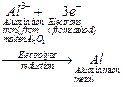
You need to login to perform this action.
You will be redirected in
3 sec
