Answer:
(i) Though hydrogen is
not a metal but even then it has been assigned a place in the activity series.
The reason is that like metals, hydrogen also has a tendency to lose electron
and forms a positive ion H+.
The metals
which lose electrons less readily than hydrogen are placed below it and the
metals which lose electrons more readily than hydrogen are placed above it in
the reactivity series of metals.
(ii)
By displacement reaction silver can be shown to be chemically less reactive
than copper or copper is more reactive than silver. If a piece of silver is
immersed in a solution of copper sulphate, no reaction will take place because
silver is less reactive than copper and will not displace copper from the
copper sulphate solution.
CuSO4(aq) + Ag(s) ®
No reaction
On
the other hand, if a copper plate is placed in a solution of silver nitrate,
copper will slowly displace silver from the solution and blue solution of
copper nitrate is formed.
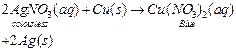 This
shows that copper is more reactive than silver.
This
shows that copper is more reactive than silver.
You need to login to perform this action.
You will be redirected in
3 sec
