Answer:
(i) The chemical bond formed by the
transfer of electrons from one atom to another is known as an ionic bond.
(ii)
An ionic bond is formed when one of the atoms can donate electrons to achieve
the inert gas electronic configuration and other atom needs electrons to
achieve the inert gas electronic configuration.
When
a metal (usually 1, 2 or 3 electrons in outermost shell) reacts with a non-metal
(usually 5, 6 or 7 electrons in outermost shell), transfer of electrons takes
place from metal atoms to the non-metal atoms and an ionic bond is formed.
There is a strong force of electrostatic attraction between metallic cation and
non-metallic anion which is responsible for the formation of ionic bond.
(iii)
Formation of magnesium chloride (MgCl2): The atomic number of
magnesium is 12. It has two electrons in its valence shell as shown below:
Mg?2, 8, 2
Magnesium,
therefore, has a tendency to lose the 2 valence electrons and in the process
attains the electronic configuration of neon.
Mg ® Mg2++
2e-
2, 8, 2 2, 8
Chlorine
(atomic number 17) has 7 electrons in the valence shell. It has a tendency to
gain one electron to complete its octet.
![]() Thus,
when magnesium and chlorine are brought together, the magnesium atom transfers
its two valence electrons to two chlorine atoms. In the process, both the atoms
acquire the stable electronic configuration of nearest inert gases. The
positively charged magnesium ion Mg2+ and negatively charged
chloride ions (Cl-) are now held together by the electrostatic force
of attraction and form ionic bond.
Mg2+- + 2Cl- ??> Mg2+ 2Cl-
or MgCl2
This process can also be shown as below:
Thus,
when magnesium and chlorine are brought together, the magnesium atom transfers
its two valence electrons to two chlorine atoms. In the process, both the atoms
acquire the stable electronic configuration of nearest inert gases. The
positively charged magnesium ion Mg2+ and negatively charged
chloride ions (Cl-) are now held together by the electrostatic force
of attraction and form ionic bond.
Mg2+- + 2Cl- ??> Mg2+ 2Cl-
or MgCl2
This process can also be shown as below:
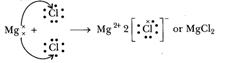
You need to login to perform this action.
You will be redirected in
3 sec
