Answer:
(i) For obtaining the
metals that are low in the reactivity series, oxides of such metals can be
reduced to metals by simply heating them in the air. For example, HgS or
cinnabar is the ore of mercury metal which on heating
changes to
HgO. This metal oxide (HgO) gets reduced to mercury metal (Hg) on further
heating.
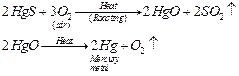 (ii) For obtaining metals that are in the middle of
reactivity series, oxides of such metals can be reduced with coke (carbon) which
acts as a reducing agent. For example, iron (III) oxide can be reduced to iron,
as follows:
(ii) For obtaining metals that are in the middle of
reactivity series, oxides of such metals can be reduced with coke (carbon) which
acts as a reducing agent. For example, iron (III) oxide can be reduced to iron,
as follows:
![]() (iii)
For obtaining metals that are high up in the reactivity series, their oxides are
reduced to metals by electrolysis.
For
example,
(iii)
For obtaining metals that are high up in the reactivity series, their oxides are
reduced to metals by electrolysis.
For
example,
![]() At
cathode:
At
cathode: ![]() At
anode:
At
anode: ![]()
You need to login to perform this action.
You will be redirected in
3 sec
