Answer:
Copper is placed above
the silver in the reactivity series which indicates that copper is more
reactive than silver. When a copper coin or strip is kept immersed in a
solution of silver nitrate (AgNO3), silver from its solution will deposit
on copper coin.
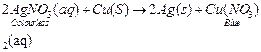 Copper
slowly displaces silver from the AgNO3 solution and the colour of solution
changes from colourless to blue due to formation of copper nitrate [Cu(NO3)2].
The copper coin will disappear and silver will precipitate out.
Copper
slowly displaces silver from the AgNO3 solution and the colour of solution
changes from colourless to blue due to formation of copper nitrate [Cu(NO3)2].
The copper coin will disappear and silver will precipitate out.
You need to login to perform this action.
You will be redirected in
3 sec
