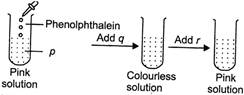
A) p \[\to \]Acid, q \[\to \]Acid, r\[\to \]Base
B) p \[\to \] Base, q\[\to \] Acid, r\[\to \] Base
C) p \[\to \] Base, q\[\to \] Base, r\[\to \]Acid
D) p \[\to \] Base, q\[\to \] Acid, r\[\to \]Acid
Correct Answer: B
Solution :
Phenolphthalein gives pink colour in basic solutions so, (p) is a base. When an acid (q) is added to this solution, it neutralises base and the solution becomes neutral. Phenolphthalein remains colourless in neutral solutions. When base (r) is added to the neutral solution, phenolphthalein again turns pink in basic solution.You need to login to perform this action.
You will be redirected in
3 sec
