| Sunil set up an experiment as shown in the figure to test a few solutions which contain hydrogen but are not categorized as acids. |
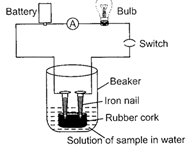
|
| (i) When a solution of glucose is put the bulb does not glow. |
| (ii) When a solution of ethanol is put the bulb does not glow. |
| (iii) When a solution of hydrochloric acid is put the bulb glows. |
| (iv) When a solution of sodium hydroxide is put the bulb does not glow. |
A) (i), (ii) and (iv) only
B) (i), (ii) and (iii) only
C) (i) and (ii) only
D) (iii) and (iv) only
Correct Answer: B
Solution :
Glucose and ethanol do not conduct electricity while \[NaOH\] and \[HCl\] conduct electricity as they produce ions in the solution.You need to login to perform this action.
You will be redirected in
3 sec
