A) \[s{{p}^{3}}\] hybridised
B) \[s{{p}^{2}}\]hybridized
C) \[sp\] hybridised
D) \[s{{p}^{2}}d\] hybridized
Correct Answer: B
Solution :
\[C{{H}_{3}}-C{{H}_{3}}\underset{\text{bond fission}}{\mathop{\xrightarrow{\text{Homolytic}}}}\,\,\underset{\text{Methyl}\,\text{free}\,\text{radicals}}{\mathop{CH_{3}^{\bullet }\,+\,CH_{3}^{\bullet }}}\,\] Free radical is formed which is \[s{{p}^{2}}\]-hybridized.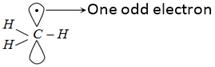
You need to login to perform this action.
You will be redirected in
3 sec
