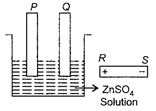
A) P must be connected to S and Q to R
B) P must be connected to Q and S to R
C) P must be connected to R and Q to S
D) P and Q must be connected to R.
Correct Answer: C
Solution :
Zinc sulphate is the salt that forms hydrogen at the cathode. As electrode P be plated with zinc, the electrode P is the anode and P must be connected to R, a positive terminal of the battery and Q is the cathode and must be connected to S.You need to login to perform this action.
You will be redirected in
3 sec
