| Rohan took 10 mL of freshly prepared iron sulphate solution in four different test tubes and added four different metal strips to each test tube as shown below: |
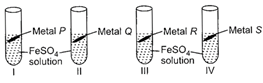
|
| In test tubes I and III, black residue was obtained while in test tubes II and IV, no change was observed. Metals P, Q, R and S could be respectively |
A) \[Al,\,Cu,\,Pb,\,Ag\]
B) \[Pb,\,Cu,\,Ag,\,Al\]
C) \[Zn,\,Al,\,Cu,\,Ag\]
D) \[Zn,\,Cu,\,Al,\,Ag\]
Correct Answer: D
Solution :
Black residue is obtained in test tubes I and III i.e., iron gets displaced in test tubes I and III. Therefore, metals P and R should be more reactive than iron. No change is observed in test tubes II and IV hence, metals Q and S should be less reactive than iron.You need to login to perform this action.
You will be redirected in
3 sec
