| Student | Colour of the solution | I | II | III | IV |
| P | Initial | Colour- less | Colour- less | Light green | Blue |
| Final | Colour- less | Colour- less | Colour- less | Colour- less | |
| Q | Initial | Colour- less | Light yellow | Light green | Blue |
| Final | Colour- less | Colour- less | Light green | Colour- less | |
| R | Initial | Colour- less | Colour- less | Light green | Blue |
| Final | Light blue | Colour- less | Colour- less | Light blue | |
| S | Initial | Light green | Colour- less | Light green | Blue |
| Final | Colour- less | Colour- less | Dark green | Colour- less |
A) P
B) Q
C) R
D) S
Correct Answer: A
Solution :
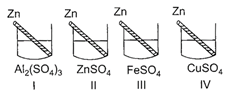
Reactivity of metals as per the reactivity series is \[Al>Zn>Fe>Cu\]. In beaker I, as Zn is less reactive than Al, it cannot displace Al from\[A{{l}_{2}}{{(S{{O}_{4}})}_{3}}\]solution hence, solution remains colourless. In beaker II, there is no effect as Zn is dipped in its own salt solution. In beaker III, as Zn is more reactive than Fe hence, it displaces Fe from \[FeS{{O}_{4}}\]solution and \[ZnS{{O}_{4}}\]is formed. Thus, colour of the solution changes from light green to colourless. \[Z{{n}_{(s)}}+\underset{Light\,green}{\mathop{FeS{{O}_{4(aq)}}}}\,\xrightarrow{{}}\underset{Colourless}{\mathop{ZnS{{O}_{4(aq)}}}}\,+F{{e}_{(s)}}\] In beaker IV, as Zn is more reactive than Cu, so it displaces Cu from \[CuS{{O}_{4}}\]solution and \[ZnS{{O}_{4}}\]is formed. Thus, colour of the solution changes from blue to colourless. \[Z{{n}_{(s)}}+\underset{Blue}{\mathop{CuS{{O}_{4(aq)}}}}\,\xrightarrow{{}}\underset{Colourless}{\mathop{ZnS{{O}_{4(aq)}}}}\,+C{{u}_{(s)}}\] Hence, the observations noted by student P are correct.You need to login to perform this action.
You will be redirected in
3 sec
