A) \[{{[Fe{{(CN)}_{6}}]}^{4-}}>{{[MnC{{l}_{4}}]}^{2-}}>{{[CoC{{l}_{4}}]}^{2-}}\]
B) \[{{[MnC{{l}_{4}}]}^{2-}}>{{[Fe{{(CN)}_{6}}]}^{4-}}>{{[CoC{{l}_{4}}]}^{2-}}\]
C) \[{{[MnC{{l}_{4}}]}^{2-}}>{{[CoC{{l}_{4}}]}^{2-}}>{{[Fe{{(CN)}_{6}}]}^{4-}}\]
D) \[{{[Fe{{(CN)}_{6}}]}^{4-}}>{{[CoC{{l}_{4}}]}^{2-}}>{{[MnC{{l}_{4}}]}^{2-}}\] (Atomic nos. \[Mn=25,\,Fe=26,\,Co=27\])
Correct Answer: C
Solution :
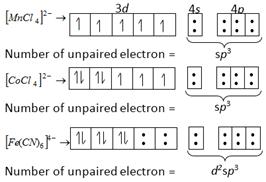 Magnetic moment \[=\sqrt{n+2}\] Where, n = number of unpaired electron i.e., greater the number of unpaired electron greater will be the paramagnetic character.
Magnetic moment \[=\sqrt{n+2}\] Where, n = number of unpaired electron i.e., greater the number of unpaired electron greater will be the paramagnetic character.
You need to login to perform this action.
You will be redirected in
3 sec
