A) 24.8 gm
B) 24 gm
C) 36.6 gm
D) 30 gm
Correct Answer: A
Solution :
Let m gm of steam get condensed into water (By heat loss). This happens in following two steps.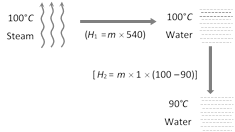 Heat gained by water (20°C) to raise it?s temperature upto 90° \[=22\times 1\times (90-20)\] Hence, in equilibrium heat lost = Heat gain Þ \[m\times 540+m\times 1\times (100-90)=22\times 1\times (90-20)\] Þ \[m=2.8\]gm The net mass of the water present in the mixture \[=22+2.8=24.8\,gm.\]
Heat gained by water (20°C) to raise it?s temperature upto 90° \[=22\times 1\times (90-20)\] Hence, in equilibrium heat lost = Heat gain Þ \[m\times 540+m\times 1\times (100-90)=22\times 1\times (90-20)\] Þ \[m=2.8\]gm The net mass of the water present in the mixture \[=22+2.8=24.8\,gm.\]
You need to login to perform this action.
You will be redirected in
3 sec
