A) Adiabatic < Isothermal < Isobaric
B) Isobaric < Adiabatic < Isothermal
C) Adiabatic < Isobaric < Isothermal
D) None of these
Correct Answer: A
Solution :
In thermodynamic process, work done is equal to the area covered by the PV curve with volume axis. Hence, according to graph shown \[{{W}_{adiabatic}}<{{W}_{isothermal}}<{{W}_{isobaric}}\]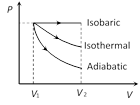
You need to login to perform this action.
You will be redirected in
3 sec
