-
question_answer1)
The energy of \[H{{e}^{+}}\] in the ground state is -54.4 eV, then the energy of \[L{{i}^{++}}\] in the first excited state will be
A)
-30.6 eV done
clear
B)
27.2 eV done
clear
C)
-13.6 eV done
clear
D)
- 27.2 eV done
clear
View Solution play_arrow
-
question_answer2)
The wavelength \[{{K}_{\alpha }}\] of X-rays for two metals 'A' and ?B? are \[\frac{4}{1875R}\] and \[\frac{1}{675R}\] respectively, where 'R' is Rydberg constant. Find the number of elements lying between A and B according to their atomic numbers
A)
3 done
clear
B)
1 done
clear
C)
4 done
clear
D)
5 done
clear
View Solution play_arrow
-
question_answer3)
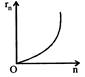
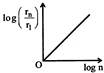
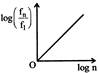
If in hydrogen atom, radius of nth Bohr orbit is \[{{r}_{n}}\], frequency of revolution of electron in \[{{n}^{th}}\] orbit is \[{{f}_{n}}\], choose the correct option.
A)
B)
C)
D)
Both and done
clear
View Solution play_arrow
-
question_answer4)
The de-Broglie wavelength associated with the electron in the \[\,n=4\] level is :
A)
\[\frac{1}{4}th\] of the de-Broglie wavelength of the electron in the ground state. done
clear
B)
four times the de-Broglie wavelength of the electron in the ground state done
clear
C)
two times the de-Broglie wavelength of the electron in the ground state done
clear
D)
half of the de-Broglie wavelength of the electron in the ground state. done
clear
View Solution play_arrow
-
question_answer5)
Excitation energy of a hydrogen like ion in its excitation state is 40.8 eV. Energy needed to remove the electron from the ion in ground state is
A)
54.4 eV done
clear
B)
13.6eV done
clear
C)
40.8 eV done
clear
D)
27.2 eV done
clear
View Solution play_arrow
-
question_answer6)
The diagram shows the energy levels for an electron in a certain atom. Which transition shown represents the emission of a photon with the most energy?

A)
I done
clear
B)
II done
clear
C)
III done
clear
D)
IV done
clear
View Solution play_arrow
-
question_answer7)
If one were to apply Bohr model to a particle of mass 'm' and charge 'q' moving in a plane under the influence of a magnetic field 'B', the energy of the charged particle in the nth level will be:
A)
\[n\left( \frac{hqB}{2\pi m} \right)\] done
clear
B)
\[n\left( \frac{hqB}{8\pi m} \right)\] done
clear
C)
\[n\left( \frac{hqB}{4\pi m} \right)\] done
clear
D)
\[n\left( \frac{hqB}{\pi m} \right)\] done
clear
View Solution play_arrow
-
question_answer8)
The acceleration of an electron in the first orbit of the hydrogen atom (z=1) is:
A)
\[\frac{{{h}^{2}}}{{{\pi }^{2}}{{m}^{2}}{{r}^{3}}}\] done
clear
B)
\[\frac{{{h}^{2}}}{8{{\pi }^{2}}{{m}^{2}}{{r}^{3}}}\] done
clear
C)
\[\frac{{{h}^{2}}}{4{{\pi }^{2}}{{m}^{2}}{{r}^{3}}}\] done
clear
D)
\[\frac{{{h}^{2}}}{4\pi {{m}^{2}}{{r}^{3}}}\] done
clear
View Solution play_arrow
-
question_answer9)
In Rutherford's experiment, the number of \[\alpha \]- particles scattered through an angle of \[60{}^\circ \]by a silver foil is 200 per minute. When the silver foil is replaced by a copper foil of the same thickness, the number of \[\alpha \]-particles scattered through an angle of \[60{}^\circ \] per minute is:
A)
\[\frac{200\times {{Z}_{Cu}}}{{{Z}_{Ag}}}\] done
clear
B)
\[200\times {{\left( \frac{{{Z}_{Cu}}}{{{Z}_{Ag}}} \right)}^{2}}\] done
clear
C)
\[200\times \frac{{{Z}_{Cu}}}{{{Z}_{Ag}}}\] done
clear
D)
\[200\times {{\left( \frac{{{Z}_{Ag}}}{{{Z}_{Cu}}} \right)}^{2}}\] done
clear
View Solution play_arrow
-
question_answer10)
An excited state of doubly ionized Lithium \[(L{{i}^{2+}})\] has an orbital radius that is about 1.33 times that of the ground state of hydrogen (H) (in Bohr's theory). The ratio of energy of the two states, \[E(L{{i}^{2+}})/E(H)\] is
A)
2.25 done
clear
B)
4.5 done
clear
C)
1 done
clear
D)
9 done
clear
View Solution play_arrow
-
question_answer11)
If elements with principal quantum number n>4 were not allowed in nature, the number of possible elements would be
A)
60 done
clear
B)
32 done
clear
C)
4 done
clear
D)
64 done
clear
View Solution play_arrow
-
question_answer12)
In the Bohr's model of hydrogen-like atom the force between the nucleus and the electron is modified as \[F=\frac{{{e}^{2}}}{4\pi {{\varepsilon }_{0}}}\left( \frac{1}{{{r}^{2}}}+\frac{\beta }{{{r}^{3}}} \right),\] where \[\beta \] is a constant. For this atom, the radius of the nth orbit in terms of the Bohr radius \[\left( {{a}_{0}}=\frac{{{\varepsilon }_{0}}{{h}^{2}}}{m\pi {{e}^{2}}} \right)\] is:
A)
\[{{r}_{n}}={{a}_{0}}n-\beta \] done
clear
B)
\[{{r}_{n}}={{a}_{0}}{{n}^{2}}+\beta \] done
clear
C)
\[{{r}_{n}}={{a}_{0}}{{n}^{2}}-\beta \] done
clear
D)
\[{{r}_{n}}={{a}_{0}}n+\beta \] done
clear
View Solution play_arrow
-
question_answer13)
Consider the spectral line resulting from the transition \[n=2\to n=1\] in the atoms and ions given below. The shortest wavelength is produced by
A)
Hydrogen atom done
clear
B)
Deuterium atom done
clear
C)
Singly ionized Helium done
clear
D)
Doubly ionized Lithium done
clear
View Solution play_arrow
-
question_answer14)
In the Bohr model of a hydrogen atom, the centripetal force is furnished by the coulomb attraction between the proton and the electron. If \[{{a}_{0}}\] is the radius of the ground state orbit, m is he mass, e is the charge on the electron and \[{{\varepsilon }_{0}}\] is the vacuum permittivity, the speed of the electron is
A)
0 done
clear
B)
\[\frac{e}{\sqrt{{{\varepsilon }_{0}}a{{ }_{0}}m}}\] done
clear
C)
\[\frac{e}{\sqrt{4\pi {{\varepsilon }_{0}}a{{ }_{0}}m}}\] done
clear
D)
\[\frac{\sqrt{4\pi {{\varepsilon }_{0}}a{{ }_{0}}m}}{e}\] done
clear
View Solution play_arrow
-
question_answer15)
A hydrogen atom and a doubly ionized lithium atom are both in the second excited state. If \[{{L}_{H}}\] and \[{{L}_{Li}}\] respectively represent their electronic angular momenta and \[{{E}_{H}}\] and \[{{E}_{Li}}\] their energies, then
A)
\[{{L}_{H}}>{{L}_{Li}}\text{ and }|{{E}_{H}}|\,>\,|{{E}_{Li}}|\] done
clear
B)
\[{{L}_{H}}={{L}_{Li}}\text{ and }|{{E}_{H}}|\,<\,|{{E}_{Li}}|\] done
clear
C)
\[{{L}_{H}}={{L}_{Li}}\text{ and }|{{E}_{H}}|\,>\,|{{E}_{Li}}|\] done
clear
D)
\[\alpha \] done
clear
View Solution play_arrow
-
question_answer16)
As per Bohr model, the minimum energy (in eV) required to remove an electron from the ground state of doubly ionized Li atom (Z=3) is
A)
1.51 done
clear
B)
13.6 done
clear
C)
40.8 done
clear
D)
122.4 done
clear
View Solution play_arrow
-
question_answer17)
Ionization energy of a hydrogen-like ion A is greater than that of another hydrogen-like ion B. If r, u, E and L represent the radius of the orbit, speed of the electron, energy of the atom and orbital angular momentum of the electron respectively then in ground state
A)
\[{{r}_{A}}>{{r}_{B}}\] done
clear
B)
\[{{u}_{A}}>{{u}_{B}}\] done
clear
C)
\[{{E}_{A}}>E{{ }_{B}}\] done
clear
D)
\[{{L}_{A}}<{{L}_{B}}\] done
clear
View Solution play_arrow
-
question_answer18)
An electron in a hydrogen atom makes a transition from first excited state to ground state. The equivalent current due to circulating electron-
A)
increases 2 times done
clear
B)
increases 4 times done
clear
C)
increases 8 times done
clear
D)
remains the same done
clear
View Solution play_arrow
-
question_answer19)
According to Bohr's theory, the time averaged magnetic field at the center (i.e. nucleus) of a hydrogen atom due to the motion of electrons in the nth orbit is proportional to : (n = principal quantum number)
A)
\[{{n}^{-4}}\] done
clear
B)
\[{{n}^{-5}}\] done
clear
C)
\[{{n}^{-3}}\] done
clear
D)
\[{{n}^{-2}}\] done
clear
View Solution play_arrow
-
question_answer20)
The momentum of hydrogen atom when a photon is emitted in a transition from \[{{n}_{i}}=10\]to \[{{n}_{f}}\] is-
A)
\[\,7\times {{10}^{-27}}\text{ }kg/ms~\] done
clear
B)
\[\,7\times {{10}^{27}}\text{ }kg/ms~\] done
clear
C)
\[\,3.5\times {{10}^{-27}}\text{ }kg/ms~\] done
clear
D)
\[\,3.5\times {{10}^{27}}\text{ }kg/ms~\] done
clear
View Solution play_arrow
-
question_answer21)
An \[\alpha \]-particle of energy 5 MeV is scattered through \[180{}^\circ \] by a fixed uranium nucleus. The distance of closest approach is of the order of
A)
\[{{10}^{-12}}cm\] done
clear
B)
\[{{10}^{-10}}cm\] done
clear
C)
\[{{10}^{-14}}cm\] done
clear
D)
\[{{10}^{-15}}cm\,\] done
clear
View Solution play_arrow
-
question_answer22)
In a hydrogen atom following the Bohr's postulates the product of linear momentum and angular momentum is proportional to \[{{(n)}^{x}}\] where 'n' is the orbit number. Then 'x' is-
A)
0 done
clear
B)
2 done
clear
C)
-2 done
clear
D)
1 done
clear
View Solution play_arrow
-
question_answer23)
A hydrogen atom makes a transition from n=2 to n=1 and emits a photon. This photon strikes a doubly ionized lithium atom (z = 3) in excited state and completely removes the orbiting electron. The least quantum number for the excited state of the ion for the process is :
A)
2 done
clear
B)
4 done
clear
C)
5 done
clear
D)
3 done
clear
View Solution play_arrow
-
question_answer24)
In the hydrogen atom, an electron makes a transition from n = 2 to n = 1. The magnetic field produced by the circulating electron at the nucleus -
A)
decreases 16 times done
clear
B)
increases 4 times done
clear
C)
decreases 4 times done
clear
D)
increases 32 times done
clear
View Solution play_arrow
-
question_answer25)
Suppose that a material emits X-rays of wavelengths\[\,\lambda {{\kappa }_{\alpha }},\lambda {{\kappa }_{\beta }},{{\lambda }_{{{L}_{\alpha }}}}\], when it is excited by fast moving electrons; the wavelengths corresponding to \[{{K}_{\alpha }},{{K}_{\beta }},{{L}_{\alpha }}\]X- rays of the material respectively. Then we can write
A)
\[\,\lambda {{\kappa }_{\beta }}=\lambda {{\kappa }_{\alpha }}+{{\lambda }_{{{L}_{\alpha }}}}\] done
clear
B)
\[\sqrt{\,\lambda {{\kappa }_{\beta }}}=\sqrt{\lambda {{\kappa }_{\alpha }}}+\sqrt{{{\lambda }_{{{L}_{\alpha }}}}}\] done
clear
C)
\[\,\frac{1}{\lambda {{\kappa }_{\beta }}}=\frac{1}{\lambda {{\kappa }_{\alpha }}}+\frac{1}{{{\lambda }_{{{L}_{\alpha }}}}}\] done
clear
D)
\[\,\frac{1}{\sqrt{\lambda {{\kappa }_{\beta }}}}=\frac{1}{\sqrt{\lambda {{\kappa }_{\alpha }}}}+\frac{1}{\sqrt{{{\lambda }_{{{L}_{\alpha }}}}}}\] done
clear
View Solution play_arrow
-
question_answer26)
Which of the following statements are true regarding Bohr's model of hydrogen atom? (I) Orbiting speed of electron decreases as it shifts to discrete orbits away from the nucleus (II) Radii of allowed orbits of electron are proportional to the principal quantum number (III) Frequency with which electrons orbit around the nucleus in discrete orbits is inversely proportional to the cube of principal quantum number (IV) Binding force with which the electron is bound to the nucleus increases as it shifts o outer orbits Select correct answer using the codes given below. Codes:
A)
I and II done
clear
B)
II and IV done
clear
C)
I, II and III done
clear
D)
II, III and IV done
clear
View Solution play_arrow
-
question_answer27)
Suppose an electron is attracted towards the k origin by a force \[\frac{k}{r}\] where A: is a constant and r is the distance of the electron from the origin. By applying Bohr model to this system, the radius of the nth orbital of the electron is found to be \['{{r}_{n}}'\] and the kinetic energy of the electron to be\['{{K}_{n}}'\]. Then winch of the following is true
A)
\[{{K}_{n}}\] independent of n, \[{{r}_{n}}\propto n\] done
clear
B)
\[{{K}_{n}}\propto \frac{1}{n},{{r}_{n}}\propto n\] done
clear
C)
\[{{K}_{n}}\propto \frac{1}{n},{{r}_{n}}\propto {{n}^{2}}\] done
clear
D)
\[{{K}_{n}}\propto \frac{1}{{{n}^{2}}},{{r}_{n}}\propto {{n}^{2}}\] done
clear
View Solution play_arrow
-
question_answer28)
Find the maximum angular speed \[(in\,\times {{10}^{16}}\,inrad/sec)\] of the electron of a hydrogen atom in a stationary orbit.
A)
2.1 done
clear
B)
4.1 done
clear
C)
8.5 done
clear
D)
11.6 done
clear
View Solution play_arrow
-
question_answer29)
Assume in specific conditions only those transitions are allowed to hydrogen atoms in which the principal quantum number n changes by 2. Find the smallest wavelength emitted by hydrogen and wavelength emitted by hydrogen in the visible range (380 nm to 780 nm). respectively (in nm)
A)
103, 487 done
clear
B)
123, 532 done
clear
C)
211, 410 done
clear
D)
320, 435 done
clear
View Solution play_arrow
-
question_answer30)
An electron in a hydrogen atom makes a transition from \[n={{n}_{1}}\] to\[n={{n}_{2}}\]. The time period of electron in the initial state is eight times that in the final state. Then which of the following statement is true?
A)
\[{{n}_{1}}=3{{n}_{2}}\] done
clear
B)
\[{{n}_{1}}=4{{n}_{2}}\] done
clear
C)
\[{{n}_{1}}=2{{n}_{2}}\] done
clear
D)
\[{{n}_{1}}=5{{n}_{2}}\] done
clear
View Solution play_arrow
-
question_answer31)
If the angular momentum of an electron in an orbit is J then the K.E. of the electron in that orbit is
A)
\[\,\frac{{{J}^{2}}}{2m{{r}^{2}}}\] done
clear
B)
\[\,\frac{Jv}{r}\] done
clear
C)
\[\,\frac{{{J}^{2}}}{2m}\] done
clear
D)
\[\,\frac{{{J}^{2}}}{2\pi }\] done
clear
View Solution play_arrow
-
question_answer32)
A neutron travelling with a velocity v and kinetic energy E has a perfectly elastic head-on collision with a nucleus of an atom of mass number A at rest. The fraction of total energy retained by the neutron is approximately
A)
\[{{\left[ \left( A-1 \right)\left( A+1 \right) \right]}^{2}}\] done
clear
B)
\[{{\left[ \left( A+1 \right)\left( A-1 \right) \right]}^{2}}\] done
clear
C)
\[{{\left[ \left( A-1 \right)/A \right]}^{2}}\] done
clear
D)
\[{{\left[ \left( A+1 \right)/A \right]}^{2}}\] done
clear
View Solution play_arrow
-
question_answer33)
In a Rutherford experiment, the number of particles scattered at \[90{}^\circ \]angle are 28 per minute then number of scattered particles at an angle \[~60{}^\circ \] and \[120{}^\circ \] will be
A)
117 per minute, 25 per minute done
clear
B)
50 per minute, 12.5 per minute done
clear
C)
100 per minute, 200 per minute done
clear
D)
112 per minute, 12.4 per minute done
clear
View Solution play_arrow
-
question_answer34)
The energy difference between the first two levels of hydrogen atom is 10.2 eV for another element of atomic number 10 and mass number 20, this will be
A)
2040 eV done
clear
B)
0.201 eV done
clear
C)
510 eV done
clear
D)
1020 eV done
clear
View Solution play_arrow
-
question_answer35)
If a proton had a radius R and the charge was uniformly distributed, calculate using Bohr theory, the total ground state energy of a H-atom when R=0.1A.
A)
-13.6 eV done
clear
B)
-5.6 eV done
clear
C)
-3.67 eV done
clear
D)
-2.67 eV. done
clear
View Solution play_arrow
-
question_answer36)
An unknown hot gas emits radiation of wavelengths 46.0 nm, 82.8 nm and 103.5 nm only. Assume that the atoms have only two excited states and the difference between consecutive energy levels decreases as energy is increased. Taking. the energy of the highest energy state to be zero, find the energies of the ground state and the first excited state.
A)
-27eV,-12eV done
clear
B)
-6eV,-3eV done
clear
C)
-HeV,-8eV done
clear
D)
-9eV,-3eV done
clear
View Solution play_arrow
-
question_answer37)
Consider 3rd orbit of \[H{{e}^{+}}\](Helium), using non-relativistic approach, the speed of electron in this orbit will be [given \[K=9\times {{10}^{9}}\]constant and h (Plank's Constant) \[6.6\times {{10}^{-34}}Js\]]
A)
\[1.46\times {{10}^{6}}m/s\] done
clear
B)
\[0.73\times {{10}^{6}}m/s\] done
clear
C)
\[3.0\times {{10}^{8}}m/s\] done
clear
D)
\[2.92\times {{10}^{6}}m/s\] done
clear
View Solution play_arrow
-
question_answer38)
A hydrogen atom in state n=6 makes two successive transitions and reaches the ground state. In the first transition a photon of 1.13 eV is emitted. Find the energy of the photon emitted in the second transition and the value of n in the intermediate state.
A)
12.1eV, n=3 done
clear
B)
6.1eV, n=4 done
clear
C)
2.1eV, n=3 done
clear
D)
1.1eV, n=5. done
clear
View Solution play_arrow
-
question_answer39)
Electrons in a certain energy level \[n={{n}_{1}}\], can emit 3 spectral lines. When they are in another energy level, \[n={{n}_{2}}\]. They can emit 6 spectral lines. The orbital speed of the electrons in the two orbits are in the ratio of
A)
4 : 3 done
clear
B)
3 : 4 done
clear
C)
2 : 1 done
clear
D)
1 : 2 done
clear
View Solution play_arrow
-
question_answer40)
An energy of 24.6 eV is required to remove one of the electrons from a neutral helium atom. The energy (in eV) required to remove both the electrons from a neutral helium atom is
A)
38.2 done
clear
B)
49.2 done
clear
C)
51.8 done
clear
D)
79.0 done
clear
View Solution play_arrow
-
question_answer41)
The wavelength of \[{{K}_{a}}\] X-rays produced by an X-ray tube is 0.76 A. Find the atomic number of the anode material of the tube?
A)
40 done
clear
B)
30 done
clear
C)
20 done
clear
D)
10 done
clear
View Solution play_arrow
-
question_answer42)
A hydrogen atom in its ground state absorbs 10.2 eV of energy The orbital angular momentum is increased by
A)
\[1.05\times {{10}^{-34}}J-s\] done
clear
B)
C)
\[2.11\times {{10}^{-34}}J-s\] done
clear
D)
\[4.22\times {{10}^{-34}}J-s\] done
clear
View Solution play_arrow
-
question_answer43)
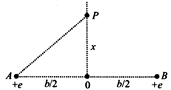
An \[\alpha \] particle passes rapidly through the exact center of a hydrogen molecule, moving on a line perpendicular to the inter nuclear axis. The distance between the nuclei is b. Where on its path does the \[\alpha \] particle experience the greatest force? (Assume that the nuclei do not move much during the passage of the \[\alpha \] particle. Also neglect the electric field of the electrons in the molecule.)
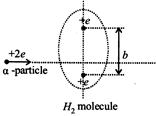
A)
\[\frac{b}{2}\] done
clear
B)
\[\frac{b}{2\sqrt{2}}\] done
clear
C)
\[\frac{b}{\sqrt{2}}\] done
clear
D)
None of these done
clear
View Solution play_arrow
-
question_answer44)
The element which has a \[{{K}_{\alpha }}\] x-rays line of wavelength 1.8 A is (\[R=1.1\times {{10}^{-7}}{{m}^{-1}},b=1\] and \[\sqrt{5/33}=0.39\])
A)
Co, Z=27 done
clear
B)
Iron, Z=26 done
clear
C)
Mn, Z=25 done
clear
D)
Ni, Z=28 done
clear
View Solution play_arrow
-
question_answer45)
Which of the following transitions in hydrogen atoms emit photons of highest frequency?
A)
n=1 to n=2 done
clear
B)
n=2 to n=6 done
clear
C)
n=6 to n=2 done
clear
D)
n=2 to n=l done
clear
View Solution play_arrow
-
question_answer46)
In the Rutherford experiment, a-particles are scattered from a nucleus as shown. Out of the four paths, which path is not possible?
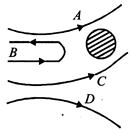
A)
D done
clear
B)
B done
clear
C)
C done
clear
D)
A done
clear
View Solution play_arrow
-
question_answer47)
Electrons are bombarded to excite hydrogen atoms and six spectral lines are observed. If \[{{E}_{g}}\] is the ground state energy of hydrogen, the minimum energy the bombarding electrons should posses is
A)
\[\frac{8{{E}_{g}}}{9}\] done
clear
B)
\[\frac{15{{E}_{g}}}{16}\] done
clear
C)
\[\frac{35{{E}_{g}}}{36}\] done
clear
D)
\[\frac{48{{E}_{g}}}{49}\] done
clear
View Solution play_arrow
-
question_answer48)
In Rutherford scattering experiment, what will be the correct angle for a-scattering for an impact parameter, b=0?
A)
\[90{}^\circ \] done
clear
B)
\[270{}^\circ \] done
clear
C)
\[0{}^\circ \] done
clear
D)
\[180{}^\circ \] done
clear
View Solution play_arrow
-
question_answer49)
If the atom \[_{100}F{{m}^{257}}\] follows the Bohr model and the radius of \[_{100}F{{m}^{257}}\] is n times the Bohr radius, then find n.
A)
100 done
clear
B)
200 done
clear
C)
4 done
clear
D)
¼ done
clear
View Solution play_arrow
-
question_answer50)
In Bohr theory of hydrogen atom, let r, v and E be the radius of orbit, speed of electron and the total energy of the electron respectively. Which of the following quantities is proportional to the quantum number n?
A)
vr done
clear
B)
rE done
clear
C)
r/E done
clear
D)
r/v done
clear
View Solution play_arrow
-
question_answer51)
The ionization energy of a hydrogen-like Bohr atom is 4 Rydbergs. Find the wavelength of radiation emitted when the electron jumps from the first excited state to the ground state: [1 Rydberg \[=2.2\times {{10}^{-18}},h=6.6\times {{10}^{-34}}Js,\] \[c=3\times {{10}^{8}}m/s\]. Bohr radius of hydrogen atom \[=5\times {{10}^{-11}}m\]
A)
\[400\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
B)
\[300\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
C)
\[500\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
D)
\[600\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
View Solution play_arrow
-
question_answer52)
An \[\alpha \]-particle of 10 MeV collides head-on with a copper nucleus (Z=29) and is deflected back. Then, the minimum distance of approach between the centers of the two is:
A)
\[8.4\times {{10}^{-15}}cm\] done
clear
B)
\[8.4\times {{10}^{-15}}m\] done
clear
C)
\[4.2\times {{10}^{-15}}m\] done
clear
D)
\[4.2\times {{10}^{-15}}cm\] done
clear
View Solution play_arrow
-
question_answer53)
In Rutherford scattering experiment, the number of a-particles scattered at \[60{}^\circ \] is \[5\times {{10}^{6}}\]. The number of a-particles scattered at \[120{}^\circ \] will be
A)
\[15\times {{10}^{6}}\] done
clear
B)
\[\frac{3}{5}\times {{10}^{6}}\] done
clear
C)
\[\frac{5}{9}\times {{10}^{6}}\] done
clear
D)
None of these done
clear
View Solution play_arrow
-
question_answer54)
Suppose potential energy between electron and proton at separation r is given by U= K In (r), where K is a constant. For such a hypothetical hydrogen atom, the ratio of energy difference between energy levels (n=1 and n=2) and (n and n=4) is
A)
1 done
clear
B)
2 done
clear
C)
3 done
clear
D)
4 done
clear
View Solution play_arrow
-
question_answer55)
The energy of a hydrogen atom in the first excited state if the potential energy is taken to be zero in the ground state.
A)
23.8 eV done
clear
B)
50.2 eV done
clear
C)
10.3 eV done
clear
D)
6.3 eV done
clear
View Solution play_arrow
-
question_answer56)
If potential energy between a proton and an electron is given by \[|U|=k{{e}^{2}}/2{{R}^{3}}\], where K is the charge of electron and R is the radius of atom, then radius of Bohr's orbit is given by (h = Planck's constant, k=constant)
A)
\[\frac{k{{e}^{2}}m}{{{h}^{2}}}\] done
clear
B)
\[\frac{6{{\pi }^{2}}}{{{n}^{2}}}\frac{k{{e}^{2}}m}{{{h}^{2}}}\] done
clear
C)
\[\frac{2\pi }{n}\frac{k{{e}^{2}}m}{{{h}^{2}}}\] done
clear
D)
\[\frac{4{{\pi }^{2}}k{{e}^{2}}m}{{{n}^{2}}{{h}^{2}}}\] done
clear
View Solution play_arrow
-
question_answer57)
In a hypothetical system, a particle of mass m and charge -3q is moving around a very heavy particle charge q. Assume that Bohr's model is applicable to this system, then velocity of mass m in the first orbit is
A)
\[\frac{3{{q}^{2}}}{2{{\varepsilon }_{0}}h}\] done
clear
B)
\[\frac{3{{q}^{2}}}{4{{\varepsilon }_{0}}h}\] done
clear
C)
\[\frac{3q}{2\pi {{\varepsilon }_{0}}h}\] done
clear
D)
\[\frac{3q}{4\pi {{\varepsilon }_{0}}h}\] done
clear
View Solution play_arrow
-
question_answer58)
The energy of an electron in an excited hydrogen atom is -3.4 eV. Then, according to Bohr's theory, the angular momentum of this electron, in Js, is
A)
\[2.11\times {{10}^{-34}}\] done
clear
B)
\[3\times {{10}^{-34}}\] done
clear
C)
\[1.055\times {{10}^{-34}}\] done
clear
D)
\[0.5\times {{10}^{-34}}\,\] done
clear
View Solution play_arrow
-
question_answer59)
Energy required for the electron excitation in \[L{{i}^{+}}\] from the first to the third Bohr orbit is:
A)
36.3 eV done
clear
B)
108.8 eV done
clear
C)
122.4eV done
clear
D)
12.1 eV done
clear
View Solution play_arrow
-
question_answer60)
Hydrogen \[{{(}_{1}}{{H}^{1}})\], Deuterium \[{{(}_{1}}{{H}^{2}})\], singly ionized Helium \[{{{{(}_{2}}{{H}^{4}})}^{+}}\] and doubly ionized lithium \[{{{{(}_{3}}L{{i}^{6}})}^{++}}\] all have one electron around the nucleus. Consider an electron transition from n=2 to n=1. If the wavelengths of emitted radiation are \[{{\lambda }_{1}},{{\lambda }_{2}},{{\lambda }_{3}}\text{ and }{{\lambda }_{4}}\] respectively then approximately which one of the following is correct?
A)
\[4{{\lambda }_{1}}=2{{\lambda }_{2}}=2{{\lambda }_{3}}={{\lambda }_{4}}\] done
clear
B)
\[{{\lambda }_{1}}=2{{\lambda }_{2}}=2{{\lambda }_{3}}={{\lambda }_{4}}\] done
clear
C)
\[{{\lambda }_{1}}={{\lambda }_{2}}=4{{\lambda }_{3}}=9{{\lambda }_{4}}\] done
clear
D)
\[{{\lambda }_{1}}=2{{\lambda }_{2}}=3{{\lambda }_{3}}=4{{\lambda }_{4}}\] done
clear
View Solution play_arrow
-
question_answer61)
Hydrogen atom is excited from ground state to another state with principal quantum number equal to 4. Then the number of spectral lines in the emission spectra will be:
A)
2 done
clear
B)
3 done
clear
C)
5 done
clear
D)
6 done
clear
View Solution play_arrow
-
question_answer62)
As an electron makes a transition from an excited state to the ground state of a hydrogen - like atom/ion:
A)
kinetic energy decreases, potential energy increases but total energy remains same done
clear
B)
kinetic energy and total energy decrease but potential energy increases done
clear
C)
its kinetic energy increases but potential energy and total energy decrease done
clear
D)
kinetic energy, potential energy and total energy decrease done
clear
View Solution play_arrow
-
question_answer63)
In a hydrogen like atom electron make transition from an energy level with quantum number n to another with quantum number (n-1). If n>>1, the frequency of radiation emitted is proportional to:
A)
\[\frac{1}{n}\] done
clear
B)
\[\frac{1}{{{n}^{2}}}\] done
clear
C)
\[\frac{1}{{}^{{{n}^{3}}}/{}_{2}}\] done
clear
D)
\[\frac{1}{{{n}^{3}}}\] done
clear
View Solution play_arrow
-
question_answer64)
A diatomic molecule is made of two masses \[{{m}_{1}}\] and \[{{m}_{2}}\] which are separated by a distance r. If we calculate its rotational energy by applying Bohr's rule of angular momentum quantization, its energy will be given by: (n is an integer)
A)
\[\frac{{{\left( {{m}_{1}}+{{m}_{2}} \right)}^{2}}{{n}^{2}}{{h}^{2}}}{2m_{1}^{2}m_{2}^{2}{{r}^{2}}}\] done
clear
B)
\[\frac{{{n}^{2}}{{h}^{2}}}{2\left( {{m}_{1}}+{{m}_{2}} \right){{r}^{2}}}\] done
clear
C)
\[\frac{2{{n}^{2}}{{h}^{2}}}{\left( {{m}_{1}}+{{m}_{2}} \right){{r}^{2}}}\] done
clear
D)
\[\frac{\left( {{m}_{1}}+{{m}_{2}} \right){{n}^{2}}{{h}^{2}}}{2{{m}_{1}}{{m}_{2}}{{r}^{2}}}\] done
clear
View Solution play_arrow
-
question_answer65)
Some energy levels of a molecule are shown in the figure. The ratio of the wavelengths \[r={{\lambda }_{1}}/{{\lambda }_{2}},\] is given by
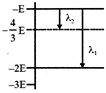
A)
\[r=\frac{3}{4}\] done
clear
B)
\[r=\frac{1}{3}\] done
clear
C)
\[r=\frac{4}{3}\] done
clear
D)
\[r=\frac{2}{3}\] done
clear
View Solution play_arrow
-
question_answer66)
Consider a 3-level system with energies \[{{E}_{1}},{{E}_{2}}\text{ and }{{E}_{3}}\] in ascending order. \[{{\lambda }_{1}},{{\lambda }_{2}}\text{ and }{{\lambda }_{3}}\] are the wavelengths of radiation corresponding to the transitions \[{{E}_{2}}\to {{E}_{1}},{{E}_{3}}\to {{E}_{2}}\text{ and }{{E}_{3}}\to {{E}_{1}}\] respectively. The wavelengths are related by
A)
\[{{\lambda }_{1}}={{\lambda }_{2}}{{\lambda }_{3}}/\left( {{\lambda }_{3}}-{{\lambda }_{2}} \right)\] done
clear
B)
\[{{\lambda }_{2}}={{\lambda }_{3}}-{{\lambda }_{1}}\] done
clear
C)
\[{{\lambda }_{2}}={{\lambda }_{1}}{{\lambda }_{3}}/\left( {{\lambda }_{1}}+{{\lambda }_{3}} \right)\] done
clear
D)
View Solution play_arrow
-
question_answer67)
Ultraviolet light of wavelengths \[{{\lambda }_{1}}\] and \[{{\lambda }_{2}}\] when allowed to fall on hydrogen atoms in their ground state is found to liberate electrons with kinetic energy \[K.{{E}_{1}}\] and \[K.{{E}_{2}}\] respectively. Find the value of Planck's constant.
A)
\[h=\left| \frac{\left( K.{{E}_{2}}-K.{{E}_{1}} \right)\left( {{\lambda }_{1}}+{{\lambda }_{2}} \right)}{C\left( {{\lambda }_{1}}-{{\lambda }_{2}} \right)} \right|\] done
clear
B)
\[h=\left| \frac{\left( K.{{E}_{1}}-K.{{E}_{2}} \right)\left( {{\lambda }_{2}}-{{\lambda }_{1}} \right)}{C{{\lambda }_{1}}{{\lambda }_{2}}} \right|\] done
clear
C)
\[h=\left| \frac{\left( K.{{E}_{1}}-K.{{E}_{2}} \right){{\lambda }_{1}}{{\lambda }_{2}}}{C\left( {{\lambda }_{2}}-{{\lambda }_{1}} \right)} \right|\] done
clear
D)
None of These done
clear
View Solution play_arrow
-
question_answer68)
In an experiment on photoelectric effect photons of wavelength 300 nm eject electrons from a metal of work function 2.25eV. A photon of energy equal to that of the most energetic electron corresponds to the following transition in the hydrogen atom:
A)
n=2 to n=1 state. done
clear
B)
n=3 to n=1 state. done
clear
C)
n=3 to n=2 state. done
clear
D)
n=4 to n=3 state. done
clear
View Solution play_arrow
-
question_answer69)
Electrons in hydrogen like atom (Z=3) make transitions from the fifth to the fourth orbit and from the fourth to the third orbit. The resulting radiations are incident normally on a metal plate and eject photoelectrons. The stopping potential for the photoelectrons ejected by the shorter wavelength is 3.95 volts. Find the stopping potential for the photoelectrons ejected by the longer wavelength, then. (Rydberg constant
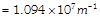
)
A)
5V done
clear
B)
2V done
clear
C)
0.754 V done
clear
D)
2.99V done
clear
View Solution play_arrow
-
question_answer70)
The ionization energy of a hydrogen like Bohr atom is 4 Rydbergs. Find the wavelength of the radiation emitted when the electron jumps from the first excited state to the ground state
A)
\[300A{}^\circ \] done
clear
B)
\[2.5\times {{10}^{-11}}m\] done
clear
C)
\[100A{}^\circ \] done
clear
D)
\[1.5\times {{10}^{-11}}m\] done
clear
View Solution play_arrow
-
question_answer71)
A neutron of kinetic energy 65eV collides in elastically with a singly ionized helium atom at rest. It is scattered at an angle of \[90{}^\circ \]with respect of its original direction. If the atom get de-excited subsequently by emitting radiation, then the impossible frequency of the emitted radiation is [Given: Mass of \[He\]atom = 4\[\times \](mass of neutron), Ionization energy of
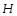
atom =13.6eV]
A)
\[1.82\times {{10}^{15}}Hz\] done
clear
B)
\[6.11\times {{10}^{15}}Hz\] done
clear
C)
\[11.67\times {{10}^{15}}Hz\] done
clear
D)
\[9.84\times {{10}^{15}}Hz\] done
clear
View Solution play_arrow
-
question_answer72)
An electron, in a hydrogen-like atom, is in an excited state. It has a total energy of-3.4 eV. The kinetic energy and the de-Broglie wavelength of the electron are respectively
A)
\[+3.4eV,0.66\times {{10}^{-9}}m\] done
clear
B)
C)
\[2.8eV,2.38\times {{10}^{-10}}m\] done
clear
D)
\[1.1eV,1.28\times {{10}^{-9}}m\] done
clear
View Solution play_arrow
-
question_answer73)
The wavelength of radiation is \[{{\lambda }_{0}}\] when an electron jumps from third to second orbit of hydrogen atom. For the electron to jump from the fourth to the second orbit of the hydrogen atom, he wavelength of radiation emitted will be
A)
\[\frac{16}{25}{{\lambda }_{0}}\] done
clear
B)
\[\frac{20}{27}{{\lambda }_{0}}\] done
clear
C)
\[\frac{27}{20}{{\lambda }_{0}}\] done
clear
D)
\[\frac{25}{16}{{\lambda }_{0}}\] done
clear
View Solution play_arrow
-
question_answer74)
In the hydrogen atom spectrum \[{{\lambda }_{3-1}}\] and \[{{\lambda }_{2-1}}\] represent wavelengths emitted due to transition from second and first excited states to the ground. state respectively. The value of \[\frac{{{\lambda }_{3-1}}}{{{\lambda }_{2-1}}}\]is
A)
27/32 done
clear
B)
32/27 done
clear
C)
4/9 done
clear
D)
9/4 done
clear
View Solution play_arrow
-
question_answer75)
The transition from the state n=4 to n=3 in a hydrogen-like atom results in ultraviolet radiation. Infrared radiation will be obtained in the transition
A)
\[2\to 1\] done
clear
B)
\[3\,\to 2\,\] done
clear
C)
\[4\to 2\] done
clear
D)
\[5\to 2\] done
clear
View Solution play_arrow
-
question_answer76)
One of the lines in the emission spectrum of \[L{{i}^{2+}}\] has the same wavelength as that of the Ist line of Lyman series in hydrogen spectrum. The electronic transition corresponding to this line is \[n=6\to 3=x\] Find the value of x.
A)
8 done
clear
B)
3 done
clear
C)
7 done
clear
D)
5 done
clear
View Solution play_arrow
-
question_answer77)
The ionization potential of H-atom is 13.6 V. When it is excited from ground state by monochromatic radiations of 970.6 A, the number of emission lines will be (according to Bohr's theory)
A)
10 done
clear
B)
8 done
clear
C)
6 done
clear
D)
4 done
clear
View Solution play_arrow
-
question_answer78)
The ratio of frequencies of the shortest wavelengths of Balmer and Lyman series of hydrogen atom is
A)
4 : 1 done
clear
B)
1 : 4 done
clear
C)
27 : 5 done
clear
D)
5 : 27 done
clear
View Solution play_arrow
-
question_answer79)
A Spectroscopic instrument can resolve two nearby wavelength \[\lambda \] and \[\lambda +\Delta \lambda \] if \[\lambda /\Delta \lambda \] is smaller than 8000. This is used to study the spectral lines of the Balmer series of hydrogen. Approximately how many lines will be resolved by the instrument?
A)
60 done
clear
B)
43 done
clear
C)
38 done
clear
D)
21 done
clear
View Solution play_arrow
-
question_answer80)
The difference between the longest wavelength line of the Balmer series and shortest wavelength line of the Lyman series for a hydrogenic atom (atomic number Z) equal to\[\Delta \lambda \]. The value of the Rydberg constant for the given atom is :
A)
\[\frac{5}{31}\frac{1}{\Delta \lambda .{{Z}^{2}}}\] done
clear
B)
\[\frac{5}{36}\frac{{{Z}^{2}}}{\Delta \lambda .}\] done
clear
C)
\[\frac{31}{5}\frac{1}{\Delta \lambda .{{Z}^{2}}}\] done
clear
D)
none of these done
clear
View Solution play_arrow
-
question_answer81)
A hydrogen atom is in ground state. Then to get six lines in emission spectrum, wavelength of incident radiation should be
A)
\[800\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
B)
\[825\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
C)
\[975\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
D)
\[01025\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
View Solution play_arrow
-
question_answer82)
The ratio of minimum to maximum wavelengths of radiation that an excited electron in a hydro- gen atom can emit while going to the ground state is
A)
1/2 done
clear
B)
Zero done
clear
C)
3/4 done
clear
D)
27/32 done
clear
View Solution play_arrow
-
question_answer83)
Whenever a photon is emitted by hydrogen in Balmer series, it is followed by another photon in Lyman series What wavelength does this latter photon correspond to?
A)
\[122\text{ }nm\] done
clear
B)
\[221\text{ }A{}^\circ \] done
clear
C)
\[\,321\text{ }nm\] done
clear
D)
\[111\text{ }A{}^\circ \] done
clear
View Solution play_arrow
-
question_answer84)
In hydrogen spectrum the wavelength of \[{{H}_{\alpha }}\] line is 656 nm, whereas in the spectrum of a distant galaxy, \[{{H}_{\alpha }}\] line wavelength is 706 nm. Estimated speed of the galaxy with respect to earth is,
A)
\[2\times {{10}^{8}}m/s\] done
clear
B)
\[2\times {{10}^{7}}m/s\] done
clear
C)
\[2\times {{10}^{6}}m/s\] done
clear
D)
\[2\times {{10}^{5}}m/s\] done
clear
View Solution play_arrow
-
question_answer85)
The first member of Balmer series of hydrogen has a wavelength of \[6563\text{ }\overset{{}^\circ }{\mathop{A}}\,\] the wavelength of its second member will be
A)
\[4861\text{ }\overset{{}^\circ }{\mathop{\text{A}}}\,\] done
clear
B)
\[6563\text{ }\overset{{}^\circ }{\mathop{\text{A}}}\,\] done
clear
C)
\[3561\overset{{}^\circ }{\mathop{\text{A}}}\,\] done
clear
D)
\[1215\overset{{}^\circ }{\mathop{\text{A}}}\,\] done
clear
View Solution play_arrow
-
question_answer86)
When a gas of hydrogen-like ions is prepared in a particular excited state X. It emits photons having wavelength equal to the wavelength of the first line of the Lyman series together with photons of five other wavelengths Identify the gas and find the principal quantum number of the state X respectively.
A)
\[H{{e}^{+}},4\] done
clear
B)
\[L{{i}^{++}},3\] done
clear
C)
\[{{H}^{+}},2\] done
clear
D)
None of these done
clear
View Solution play_arrow
-
question_answer87)
Taking Rydberg's constant \[R=1.097\times {{10}^{7}}m\], first and second wavelength of Balmer series in hydrogen spectrum is
A)
\[2000\text{ }\overset{\text{o}}{\mathop{\text{A }\!\!~\!\!\text{ }}}\,,\text{ }3000\text{ }\overset{\text{o}}{\mathop{\text{A }\!\!~\!\!\text{ }}}\,~\] done
clear
B)
\[\text{1575 }\overset{\text{o}}{\mathop{\text{A }\!\!~\!\!\text{ }}}\,\text{, }\!\!~\!\!\text{ 2960 }\overset{\text{o}}{\mathop{\text{A }\!\!~\!\!\text{ }}}\,\] done
clear
C)
\[\text{6529 }\overset{\text{o}}{\mathop{\text{A}}}\,\text{, }\,\text{4280 }\overset{\text{o}}{\mathop{\text{A }\!\!~\!\!\text{ }}}\,\] done
clear
D)
\[6552\text{ }\overset{\text{o}}{\mathop{\text{A }\!\!~\!\!\text{ }}}\,,\text{ }4863\text{ }\overset{\text{o}}{\mathop{\text{A }\!\!~\!\!\text{ }}}\,~~\] done
clear
View Solution play_arrow
-
question_answer88)
The energy of electron in the nth orbit of hydrogen atom is expressed as \[{{E}_{n}}=-\frac{13.6}{{{n}^{2}}}eV.\] The shortest and longest wavelength of Lyman series will be
A)
\[910\overset{\text{o}}{\mathop{\text{A}}}\,,\,\,1213\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
B)
\[5463\overset{\text{o}}{\mathop{\text{A}}}\,,\,\,7858\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
C)
\[1315\text{ }\overset{\text{o}}{\mathop{\text{A}}}\,,\text{ }1530\text{ }\overset{\text{o}}{\mathop{\text{A}}}\,~~\] done
clear
D)
None of these done
clear
View Solution play_arrow
-
question_answer89)
A 12.5 eV electron beam is used to bombard gaseous hydrogen at room temperature. Hydrogen atoms would be excited up to nth energy level the hence the wavelength obtained first member of Balmer series is\[{{\lambda }_{B}}\], then.
A)
\[{{\lambda }_{B}}=1415\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
B)
\[{{\lambda }_{B}}=1215\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
C)
\[{{\lambda }_{B}}=6563\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
D)
\[{{\lambda }_{B}}=8523\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
View Solution play_arrow
-
question_answer90)
If the wavelength of the first line of the Balmer series in the hydrogen spectrum is K, then the wavelength of the first line of the Lyman series is
A)
\[\left( 27/5 \right)\lambda \] done
clear
B)
\[\left( 5/27 \right)\lambda \] done
clear
C)
\[\left( 32/27 \right)\lambda \] done
clear
D)
\[\left( 27/32 \right)\lambda \] done
clear
View Solution play_arrow
-
question_answer91)
One of the lines in the emission spectrum of \[L{{i}^{2+}}\] has the same wavelength as that of the 2nd line of Balmer series in hydrogen spectrum. The electronic transition corresponding to this line is
A)
\[n=4\to n=2\] done
clear
B)
\[n=8\to n=2\] done
clear
C)
\[n=8\to n=4\] done
clear
D)
\[n=12\to n=6.3\] done
clear
View Solution play_arrow
-
question_answer92)
Hydrogen atom excites energy level from fundamental state to n=3. Number of spectral lines according to Bohr, is
A)
4 done
clear
B)
3 done
clear
C)
1 done
clear
D)
2 done
clear
View Solution play_arrow
-
question_answer93)
When an electron in a hydrogen atom makes a transition from 2nd excited stated to ground state it emits a photon of frequency/ The frequency of photon emitted when an electron of \[L{{i}^{++}}\] makes transition from Ist excited state to ground state is
A)
\[\frac{243}{32}f\] done
clear
B)
\[\frac{141}{32}f\] done
clear
C)
\[\frac{81}{32}f\] done
clear
D)
\[\frac{63}{32}f\] done
clear
View Solution play_arrow
-
question_answer94)
The photon radiated from hydrogen corresponding to 2nd line of Lyman series is absorbed by a hydrogen like atom X in 2nd excited state. As a result the hydrogen like atom X makes a transition to nth orbit. Then -
A)
\[\text{X=H}{{\text{e}}^{\text{+}}},\,\,\text{n=4}\] done
clear
B)
\[X=L{{i}^{++}},\,\,n=6\] done
clear
C)
\[X=H{{e}^{+}},n=6\] done
clear
D)
\[X=L{{i}^{++}},n=9\] done
clear
View Solution play_arrow
-
question_answer95)
A Spectroscopic instrument can resolve two nearby wavelength \[\lambda \] and \[\lambda +\Delta \lambda \]if \[\lambda /\Delta \lambda \] is smaller than 8000. This is used to study the spectral lines of the Balmer series of hydrogen. Approximately how many lines will be resolved by the instrument?
A)
60 done
clear
B)
43 done
clear
C)
38 done
clear
D)
21 done
clear
View Solution play_arrow
-
question_answer96)
If the series limit wavelength of Lyman series for the hydrogen atom is \[912\text{ }\overset{\text{o}}{\mathop{\text{A}}}\,\], then the series limit wavelength for Balmer series of hydrogen atoms is
A)
\[912\text{ }\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
B)
\[912\text{ }\times \text{2}\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
C)
\[912\text{ }\times 4\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
D)
\[\frac{912}{2}\overset{\text{o}}{\mathop{\text{A}}}\,\] done
clear
View Solution play_arrow
-
question_answer97)
The third line of the Balmer series spectrum of a hydrogen like ion of atomic number Z equals to 108.5 nm. Then Z is
A)
2 done
clear
B)
5 done
clear
C)
3 done
clear
D)
6 done
clear
View Solution play_arrow
-
question_answer98)
The ground state energy of hydrogen atom is -13.6 eV. If an electron makes a transition from an energy level - 0.85 eV to -1.51 eV, calculate the wavelength X of the spectral line emitted.
A)
\[1.88\times {{10}^{-6}}m\] done
clear
B)
\[1.87\times {{10}^{-10}}m\] done
clear
C)
\[1.66\times {{10}^{-9}}m\] done
clear
D)
\[2.36\times {{10}^{-9}}m\] done
clear
View Solution play_arrow
-
question_answer99)
If the electron revolving around the nucleus in a radius 'r' with orbital speed 'v' has magnetic moment evr/2. Hence, using Bohr's postulate of the quantization of angular momentum obtain the magnetic moment (M) of hydrogen atom in its ground state and current (I) due to revolution of electron.
A)
\[M=\frac{eh}{4\pi m},I=\frac{eV}{2\pi r}\] done
clear
B)
\[M=\frac{2eh}{5\pi m},I=\frac{eV}{4\pi r}\] done
clear
C)
\[M=\frac{h}{\pi m},I=\frac{e}{\pi r}\] done
clear
D)
\[M=\frac{eh}{\pi m},I=\frac{eV}{\pi r}\] done
clear
View Solution play_arrow
-
question_answer100)
If radiation corresponding to first line of "Balmer series" of \[H{{e}^{+}}\] ion knocked out electron from 1st excited state of H atom, the kinetic energy of ejected electron from H atom would be (eV) - [Given \[{{E}_{n}}=-\frac{{{Z}^{2}}}{{{n}^{2}}}\left( 13.6eV \right)\]]
A)
4.155 eV done
clear
B)
8.310 eV done
clear
C)
2.515 eV done
clear
D)
5.550 eV done
clear
View Solution play_arrow
 done
clear
done
clear
 done
clear
done
clear
 done
clear
done
clear
 done
clear
done
clear
