-
question_answer1)
The nuclear radius of \[_{8}{{O}^{16}}\] is \[3\times {{10}^{-15}}\]. If an atomic mass unit is \[1.67\times {{10}^{-27}}kg\], then the nuclear density is approximately
A)
\[2.35\times {{10}^{17}}g/c{{m}^{-3}}\] done
clear
B)
\[2.35\times {{10}^{17}}kg/c{{m}^{-3}}\] done
clear
C)
\[2.35\times {{10}^{17}}c{{m}^{-3}}\] done
clear
D)
\[2.35\times {{10}^{17}}kg\,\,m{{m}^{-3}}\] done
clear
View Solution play_arrow
-
question_answer2)
If the nuclear radius of \[^{27}Al\] is 3.6 Fermi, the approximate nuclear radius of \[^{64}Cu\] in Fermi is
A)
4.8 done
clear
B)
3.6 done
clear
C)
2.4 done
clear
D)
1.2 done
clear
View Solution play_arrow
-
question_answer3)
The nuclear radius of a nucleus with nucleon number 16 is \[3\times {{10}^{-15}}\text{ }m\]. Then, the nuclear radius of a nucleus with nucleon number 128 is
A)
\[3\times {{10}^{-15}}m\] done
clear
B)
\[1.5\times {{10}^{-15}}m\] done
clear
C)
\[6\times {{10}^{-15}}m\] done
clear
D)
\[4.5\times {{10}^{-15}}m\] done
clear
View Solution play_arrow
-
question_answer4)
Atomic weight of boron is 10.81 and it has two isotopes \[_{5}{{B}^{10}}\] and\[_{5}{{B}^{11}}\]. Then ratio of \[_{5}{{B}^{10}}{{:}_{5}}{{B}^{11}}\] in nature would be
A)
19 : 81 done
clear
B)
10 : 11 done
clear
C)
15 : 16 done
clear
D)
81 : 19 done
clear
View Solution play_arrow
-
question_answer5)
If the nucleus \[_{13}^{27}Al\] has nuclear radius of about 3.6 fm, then \[_{32}^{125}Te\] would have its radius approximately as
A)
9.6 fm done
clear
B)
12.0 fm done
clear
C)
4.8 fm done
clear
D)
6.0 fm. done
clear
View Solution play_arrow
-
question_answer6)
The radius of germanium \[\left( Ge \right)\] nuclide is measured to be twice the radius of \[_{4}^{9}Be\]. The number of nucleons in \[Ge\] are
A)
74 done
clear
B)
75 done
clear
C)
72 done
clear
D)
73 done
clear
View Solution play_arrow
-
question_answer7)
Calculate binding energy of \[_{92}{{U}^{238}}\]. Given \[M({{U}^{238}})=238.050783\,amu\]\[{{m}_{n}}=1.008665\,amu\] and \[{{m}_{p}}=1.007825\,amu\]
A)
801.7MeV done
clear
B)
18.7 MeV done
clear
C)
0.7 MeV done
clear
D)
1801.7 MeV. done
clear
View Solution play_arrow
-
question_answer8)
The binding energy of deuteron \[(_{1}^{2}H)\] is 1.15 A MeV per nucleon and an alpha particle \[(_{2}^{4}He)\]has a binding energy of 7.1 MeV per nucleon. Then in the reaction \[_{1}^{2}He+_{1}^{2}He\to _{2}^{2}He+Q\] the energy released Q is:
A)
5.95 MeV done
clear
B)
26.1 MeV done
clear
C)
23.8 MeV done
clear
D)
289.4 MeV done
clear
View Solution play_arrow
-
question_answer9)
Binding energy per nucleon vs mass number curve for nuclei is shown in the Figure. W, X, F and Z are four nuclei indicated on the curve. The process that would release energy is
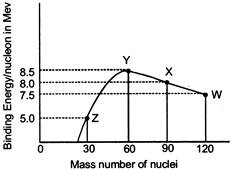
A)
\[Y\to 2Z~\] done
clear
B)
\[W\to X+Z\,\] done
clear
C)
\[W\to 2Y\] done
clear
D)
\[X\to Y+Z\] done
clear
View Solution play_arrow
-
question_answer10)
A proton is bombarded on a stationary Lithium nucleus. As a result of collision two \[\alpha \]-particles are produced. The direction of motion of the \[\alpha \]- particles with the initial direction of motion makes an angle\[{{\cos }^{-1}}\frac{1}{4}\]. If B.E./Nucleon for \[L{{i}^{7}}\] and \[\,H{{e}^{4}}\] are 5.60 MeV and 7.06 MeV respectively, then:
A)
Kinetic energy of striking proton is 17.28 MeV done
clear
B)
Kinetic energy of striking proton is 8.64 MeV done
clear
C)
Kinetic energy of striking proton is 4.32 MeV done
clear
D)
Kinetic energy of striking proton is 2.16 MeV done
clear
View Solution play_arrow
-
question_answer11)
The binding energy per nucleon of \[^{10}X\] is 9 MeV and that of \[^{11}X\] is 7.5 MeV where X represents an element. The minimum energy required to remove a neutron from \[^{11}X\] is
A)
7.5 MeV done
clear
B)
2.5 MeV done
clear
C)
8 MeV done
clear
D)
0.5 MeV done
clear
View Solution play_arrow
-
question_answer12)
The binding energies per nucleon for a deuteron and an \[\alpha \]-particle are \[{{x}_{1}}\,\] and \[{{x}_{2}}\] respectively. What will be the energy Q released in the reaction \[_{1}{{H}^{2}}{{+}_{1}}{{H}^{2}}{{\to }_{2}}{{H}^{4}}+Q\]
A)
\[4\left( {{x}_{1}}+{{x}_{2}} \right)\] done
clear
B)
\[4\left( {{x}_{2}}-{{x}_{1}} \right)\] done
clear
C)
\[2\left( {{x}_{1}}+{{x}_{2}} \right)\] done
clear
D)
\[2\left( {{x}_{2}}-{{x}_{1}} \right)\] done
clear
View Solution play_arrow
-
question_answer13)
The masses of neutron and proton are 1.0087 a.m.u. and 1.0073 a.m.u. respectively. If the neutrons and protons combine to form a helium nucleus (alpha particles) of mass 4.0015 a.m.u the binding energy of the helium nucleus will be \[\left( 1\text{ }a.m.u.=931MeV \right)\]
A)
28.4 MeV done
clear
B)
20.8 MeV done
clear
C)
27.3 MeV done
clear
D)
14.2 MeV done
clear
View Solution play_arrow
-
question_answer14)
The mass of \[_{7}{{N}^{15}}\] is 15.00011 amu, mass of \[_{8}{{O}^{16}}\] is 15.99492 amu and \[mp=1.00783\text{ }amu\] Determine binding energy of last proton of\[_{8}{{O}^{16}}\].
A)
2.13 MeV done
clear
B)
0.13 MeV done
clear
C)
10 MeV done
clear
D)
12.13 MeV done
clear
View Solution play_arrow
-
question_answer15)
A heavy nucleus having mass number 200 gets disintegrated into two small fragments of mass number 80 and 120. If binding energy per nucleon for parent atom is 6.5 MeV and for daughter nuclei is 7 MeV and 8 MeV respectively, then the energy released in the decay will be -
A)
200 MeV done
clear
B)
-220 MeV done
clear
C)
220 MeV done
clear
D)
180 MeV done
clear
View Solution play_arrow
-
question_answer16)
If the binding energy per nucleon in \[_{3}^{7}Li\] and \[_{2}^{4}He\] nuclei are 5.60 MeV and 7.06 MeV respectively, then in the reaction \[p+_{3}^{7}Li\xrightarrow{{}}2_{2}^{4}He\] energy of proton must be
A)
28.24 MeV done
clear
B)
17.28 MeV done
clear
C)
1.46MeV done
clear
D)
39.2MeV done
clear
View Solution play_arrow
-
question_answer17)
A gamma ray creates an electron-positron pair. If the rest mass energy of an electron is 0.5 MeV and the total kinetic energy of the electron-positron pair is 0.78 MeV, then the energy of the gamma ray must be
A)
0.78 MeV done
clear
B)
1.78 MeV done
clear
C)
1.28 MeV done
clear
D)
0.28 MeV done
clear
View Solution play_arrow
-
question_answer18)
If the total binding energies of \[_{1}^{2}H\], \[_{2}^{4}He\], \[_{26}^{56}Fe\] & \[_{92}^{235}U\] nuclei are 2.22, 28.3, 492 and 1786 MeV respectively, identify the most stable nucleus of the following
A)
\[_{26}^{56}Fe\] done
clear
B)
\[_{1}^{2}H\] done
clear
C)
\[_{92}^{235}U\] done
clear
D)
\[_{2}^{4}He\] done
clear
View Solution play_arrow
-
question_answer19)
The nuclear fusion reaction\[2{{H}^{2}}{{\to }_{2}}H{{e}^{4}}+\text{Energy }\], is proposed to be used for the production of industrial power. Assuming the efficiency of process for production of power is 20%, find the ass of the deuterium required approximately for a duration of 1 year. Given mass of \[_{1}{{H}^{2}}\] nucleus = 2.0141 a.m.u and mass of\[_{2}H{{e}^{4}}\] nuclei = 4.0026 a.m.u and 1 a.m.u. = 31 MeV
A)
165kg done
clear
B)
138kg done
clear
C)
180kg done
clear
D)
60kg done
clear
View Solution play_arrow
-
question_answer20)
Two deuterons undergo nuclear fusion to form a Helium nucleus. Energy released in this process is : (given binding energy per nucleon for deuteron=1. 1 MeV and for helium=7.0 MeV)
A)
30.2 MeV done
clear
B)
32.4 MeV done
clear
C)
23.6 MeV done
clear
D)
25.8 MeV done
clear
View Solution play_arrow
-
question_answer21)
In the nuclear fusion reaction \[_{1}^{2}H+_{1}^{3}H\to _{2}^{4}He+n\] given that the repulsive potential energy between the two nuclei is- \[\sim 7.7\times {{10}^{-14}}J\], the temperature at which the gases must be heated to initiate the reaction is nearly [Boltzmann's Constant \[k=1.38\times {{10}^{-23}}J/K\]]
A)
\[{{10}^{7}}K\] done
clear
B)
\[{{10}^{5}}K\] done
clear
C)
\[{{10}^{3}}K\] done
clear
D)
\[{{10}^{9}}K\] done
clear
View Solution play_arrow
-
question_answer22)
When Uranium is bombarded with neutrons, it undergoes fission. The fission reaction can be written as: \[_{92}{{U}^{235}}{{+}_{0}}{{n}^{1}}{{\to }_{56}}B{{a}^{141}}+{{\,}_{36}}K{{r}^{92}}+3x+Q\] Where three particles named x are produced and energy Q is released. What is the name of the particles?
A)
electron done
clear
B)
\[\alpha \]-particle done
clear
C)
neutron done
clear
D)
neutrino done
clear
View Solution play_arrow
-
question_answer23)
A reactor converts 100% of given mass into energy and that it operates at a power of \[9\times {{10}^{7}}\] watt. The mass of the fuel consumed in 30 min in the reactor will be:
A)
\[12\times {{10}^{-3}}kg\] done
clear
B)
\[25\times {{10}^{-8}}kg\] done
clear
C)
\[18\times {{10}^{-7}}kg\] done
clear
D)
\[11\times {{10}^{-4}}kg\] done
clear
View Solution play_arrow
-
question_answer24)
If a star can convert all the He nuclei completely into oxygen nuclei, the energy released per oxygen nuclei is [Mass of \[He\] nucleus is 4.0026 amu and mass of Oxygen nucleus is 15.9994 mu]
A)
7.6 MeV done
clear
B)
56.12 MeV done
clear
C)
10.24 MeV done
clear
D)
23.9 MeV done
clear
View Solution play_arrow
-
question_answer25)
Determine the power output of a \[_{92}{{U}^{235}}\] reactor if it takes 30 days to use 2kg of fuel. Energy released per fission is 200 MeV and \[N=6.023\times {{10}^{26}}\]per kilo mole.
A)
63.28 MW done
clear
B)
3.28 MW done
clear
C)
0.6 MW done
clear
D)
50.12 MW done
clear
View Solution play_arrow
-
question_answer26)
In the sun about 4 billion kg of matter is converted to energy each second. The power output of the sun in watt is
A)
\[~3.6\times {{10}^{26}}\] done
clear
B)
\[0.36\times {{10}^{26}}\] done
clear
C)
\[36\times {{10}^{26}}\] done
clear
D)
\[0.0036\times {{10}^{26}}\] done
clear
View Solution play_arrow
-
question_answer27)
For fission of uranium \[_{95}^{235}U\], one needs
A)
very fast neutrons (a few electrons volts) done
clear
B)
low energy neutrons (\[\approx \]0.04 eV at 300K) done
clear
C)
a moderator making neutrons slow done
clear
D)
both and done
clear
View Solution play_arrow
-
question_answer28)
Imagine that a reactor converts all given mass into energy and that it operates at a power level of 109 watt. The mass of the fuel consumed per hour in the reactor will be : (velocity of light, c is \[3\times {{10}^{8}}m/s\])
A)
\[0.96\text{ }gm\] done
clear
B)
\[0.8\text{ }gm\,\] done
clear
C)
\[4\times {{10}^{-2}}gm~~~~~\] done
clear
D)
\[6.6\times {{10}^{-5}}gm\] done
clear
View Solution play_arrow
-
question_answer29)
Consider the following reaction \[_{1}{{H}^{2}}{{+}_{1}}{{H}^{2}}{{\to }_{2}}{{H}^{4}}+Q.\] If \[m\,{{(}_{1}}{{H}^{2}})=2.014\,\,lamu;\] \[m\,{{(}_{2}}{{H}^{4}})=4.0024\,\,lamu.\] The energy Q released (in MeV) in this fusion reaction is
A)
12 done
clear
B)
6 done
clear
C)
24 done
clear
D)
48 done
clear
View Solution play_arrow
-
question_answer30)
If 200 MeV energy is released in the fission of a single \[{{U}^{235}}\] nucleus, the number of fissions required per second to produce 1 kilowatt power shall be(Given \[\,1eV=\text{ }1.6\times {{10}^{-19}}J\])
A)
\[3.125\times {{10}^{13}}\] done
clear
B)
\[\,3.125\times {{10}^{14}}\] done
clear
C)
\[3.125\times {{10}^{15}}\] done
clear
D)
\[3.125\times {{10}^{16}}\] done
clear
View Solution play_arrow
-
question_answer31)
A radioactive sample \[{{S}_{1}}\] having an activity \[5\mu Ci\] has twice the number of nuclei as another sample \[{{S}_{2}}\] which has an activity of \[10\mu Ci\]. The half-lives of \[{{S}_{1}}\] and \[{{S}_{2}}\] can be
A)
20 years and 5 years, respectively done
clear
B)
20 years and 10 years, respectively done
clear
C)
10 years each done
clear
D)
5 years each done
clear
View Solution play_arrow
-
question_answer32)
A mixture consists of two radioactive materials \[{{A}_{1}}\] and \[{{A}_{2}}\] with half-lives of 20 s and 10 s respectively. Initially the mixture has 40 g of \[{{A}_{1}}\] and 160 g of \[{{A}_{2}}\]. The amount of the two in the mixture will become equal after
A)
60s done
clear
B)
80s done
clear
C)
20s done
clear
D)
40s done
clear
View Solution play_arrow
-
question_answer33)
Two radioactive materials A and B have decay constant \[\frac{13}{7}\] units and \[\frac{19}{14}\] units respectively. Directly both have the same number of nuclei. The time (in same system of units), after which the ratio of their remaining nuclei will be 1/e is
A)
2 done
clear
B)
3 done
clear
C)
4 done
clear
D)
5 done
clear
View Solution play_arrow
-
question_answer34)
A radioactive sample contains 10 3 kg each of two nuclear species A and B with half-life 4 days and 8 days respectively The ratio of the amounts of A and B after a period of 16 days is
A)
1 : 2 done
clear
B)
4 : 1 done
clear
C)
1 : 4 done
clear
D)
2 :1 done
clear
View Solution play_arrow
-
question_answer35)
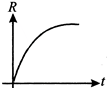
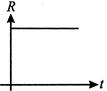
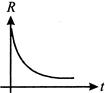
A radioactive nucleus a decays to a stable nucleus ?Y?. Then the graph of rate of formation of ?Y? against time ?t? will be
A)
B)
C)
D)
View Solution play_arrow
-
question_answer36)
A radioactive material of half-life ln2 was produced in a nuclear reactor. Consider two different instants A and B. The number of undecayed nuclei at instant B was twice of that of instant A. If the activities at instants A and B are \[{{A}_{1}}\] and \[{{A}_{2}}\] respectively then the difference in the age of the sample at these instants equals.
A)
\[\left| \ell n\left( \frac{2{{A}_{1}}}{{{A}_{2}}} \right) \right|\] done
clear
B)
\[\ell n2\left| \ell n\left( \frac{{{A}_{1}}}{{{A}_{2}}} \right) \right|\] done
clear
C)
\[\left| \ell n\left( \frac{{{A}_{1}}}{2{{A}_{2}}} \right) \right|\] done
clear
D)
\[\ell n2\left| \ell n\left( \frac{{{A}_{1}}}{{{A}_{2}}} \right) \right|\] done
clear
View Solution play_arrow
-
question_answer37)
An archaeologist analyses the wood in a prehistoric structure and finds that \[{{C}^{14}}\] (Half-life = 5700 years) to \[{{C}^{12}}\]is only one-fourth of that found in the cells of buried plants. The age of the wood is about
A)
5700 years done
clear
B)
2850 years done
clear
C)
11,400 years done
clear
D)
22,800 years done
clear
View Solution play_arrow
-
question_answer38)
Half-lives for \[\alpha \] and \[\beta \] emission of a radioactive material are 16 years and 48 years respectively. When material decays giving \[\alpha \] and \[\beta \] emission simultaneously, time in which 3/4th material decays is
A)
29 years done
clear
B)
24 years done
clear
C)
64 years done
clear
D)
12 years done
clear
View Solution play_arrow
-
question_answer39)
Actinium 231,\[^{231}A{{C}_{89}}\], emit in succession two \[\beta \] particles, four \[\alpha \]-particles, one \[\beta \] and one \[\alpha \] plus several \[\gamma \] rays. What is the resultant isotope?
A)
\[^{221}A{{u}_{79}}\] done
clear
B)
\[^{221}P{{b}_{82}}\] done
clear
C)
\[^{211}A{{u}_{79}}\] done
clear
D)
\[^{211}P{{b}_{82}}\] done
clear
View Solution play_arrow
-
question_answer40)
A radioactive source in the form of metal sphere of diameter \[{{10}^{-3}}\text{ }m\] emits beta particle at a constant rate of \[6.25\times {{10}^{10}}\] particles per second. If the source is electrically insulated, how long will it take for its potential to rise by 1.0 volt, assuming that 80% of the emitted beta particles escape from the source?
A)
\[6.95\text{ }\mu \text{ }sec\] done
clear
B)
\[\,0.95\text{ }\mu \text{ }sec\] done
clear
C)
\[1.95\mu \text{ }sec\,\] done
clear
D)
\[2.15\text{ }\mu \text{ }sec\] done
clear
View Solution play_arrow
-
question_answer41)
A radioactive nucleus undergoes a series of decay according to the scheme \[A\xrightarrow{a}{{A}_{1}}\xrightarrow{\beta }{{A}_{2}}\xrightarrow{\alpha }{{A}_{3}}\xrightarrow{\gamma }{{A}_{4}}\] If the mass number and atomic number of 'A' are 180 and 72 respectively, then what are these numbers for \[{{A}_{4}}\]
A)
172 and 69 done
clear
B)
174 and 70 done
clear
C)
176 and 69 done
clear
D)
176 and 70 done
clear
View Solution play_arrow
-
question_answer42)
` Half lives of two isotopes X and Y of a material are known to be \[2\times {{10}^{9}}years\] and \[4\times {{10}^{9}}years\,\]respectively If a planet was formed with equal number of these isotopes, then the current age of planet, given that currently the material has 20% of X and 80% of Y by number, will be
A)
\[2\times {{10}^{9}}years\] done
clear
B)
\[4\times {{10}^{9}}years\,\] done
clear
C)
\[6\times {{10}^{9}}years\] done
clear
D)
\[8\times {{10}^{9}}years\] done
clear
View Solution play_arrow
-
question_answer43)
At radioactive equilibrium, the ratio between the atoms of two radioactive elements (X) and (Y) as found to be \[3.2\times {{10}^{9}}:1\] respectively If half-life of the element is \[1.6\times {{10}^{10}}\] years, then half-life of the element W would be
A)
\[3.2\times {{10}^{9}}years\] done
clear
B)
\[5\times {{10}^{9}}years\] done
clear
C)
\[1.6\times {{10}^{10}}years\] done
clear
D)
5 years done
clear
View Solution play_arrow
-
question_answer44)
There are two radioactive substances A and B. Decay constant of B is two times that of A. Initially, both have equal number of nuclei. After n half-lives of 4, rate of disintegration of both are equal. The value of n is
A)
4 done
clear
B)
2 done
clear
C)
1 done
clear
D)
5 done
clear
View Solution play_arrow
-
question_answer45)
A 280 days old radioactive substance shows an activity of 6000 dps, 140 days later its activity becomes 3000 dps. What was its initial activity?
A)
20000 dps done
clear
B)
24000 dps done
clear
C)
12000 dps done
clear
D)
6000 dps done
clear
View Solution play_arrow
-
question_answer46)
A radioactive element X converts into another stable element Y. Half-life of X is 2 hrs. Initially only X is present. After time t, the ratio of atoms of X and Y is found to be 1 : 4, then t in hours is
A)
2 done
clear
B)
4 done
clear
C)
between 4 and 6 done
clear
D)
6 done
clear
View Solution play_arrow
-
question_answer47)
For a radioactive sample the counting rate changes from 6520 counts/minute to 3260 counts minute in 2 minutes. Determine the decay constant.
A)
1.78 per sec done
clear
B)
0.78 per sec done
clear
C)
2.78 per sec done
clear
D)
5.78 per sec done
clear
View Solution play_arrow
-
question_answer48)
Consider a radioactive material of half-life 1.0 minute. If (me of the nuclei decays now, the next one will decay
A)
after 1 minute done
clear
B)
after \[\frac{1}{{{\log }_{e}}2}\] minute done
clear
C)
after \[\frac{1}{N}\] minute, where N is the number of nuclei present at that moment done
clear
D)
after any time done
clear
View Solution play_arrow
-
question_answer49)
After 150 days, the activity of a radioactive sample is 5000 dps. The activity becomes 2500 dps after another 75 days. The initial activity of the sample is
A)
20000 dps done
clear
B)
40000 dps done
clear
C)
7500 dps done
clear
D)
10000 dps done
clear
View Solution play_arrow
-
question_answer50)
If a radioactive material contains 0.1 mg of \[T{{h}^{234}}\]how much of it will remain unchanged after 120 days. Given Half-life is 24 days
A)
\[1.12\text{ }\mu g\] done
clear
B)
\[0.1\text{ }\mu g\] done
clear
C)
\[3.125\text{ }\mu g\] done
clear
D)
\[125\text{ }\mu \text{g}.\] done
clear
View Solution play_arrow
-
question_answer51)
Consider \[\alpha \] particles, \[\beta \] particles and \[\gamma \] - rays, each having an energy of 0.5 MeV. In increasing order of penetrating powers, the radiations are:
A)
\[\alpha ,\,\,\beta ,\,\,\gamma \] done
clear
B)
\[\alpha ,\,\,\beta ,\,\,\gamma \] done
clear
C)
\[\beta ,\,\,\gamma ,\,\,\alpha \] done
clear
D)
\[\gamma ,\,\,\beta ,\,\,\alpha \] done
clear
View Solution play_arrow
-
question_answer52)
The activity of a radioactive sample is measured as \[{{N}_{0}}\] counts per minute at t=0 and \[{{N}_{0}}/e\] counts per minute at t=5 minutes. The time (in minutes) at which the activity reduces to half its value is
A)
\[{{\log }_{e}}2/5\] done
clear
B)
\[\frac{5}{{{\log }_{e}}2}\] done
clear
C)
\[5{{\log }_{10}}2\] done
clear
D)
\[5{{\log }_{e}}2\] done
clear
View Solution play_arrow
-
question_answer53)
A sample of radioactive material decays simultaneously by two processes A and B with half-lives 1/2 and 1/4 hr. respectively. For first half our it decays with the process A, next one hour with the process B and for further half an hour with both A and B. If originally there were no nuclei, the number of nuclei after 2 hour of such decay is \[{{N}_{0}}{{\left( \frac{1}{2} \right)}^{x}}\] then find the value of x.
A)
5 done
clear
B)
4 done
clear
C)
3 done
clear
D)
8 done
clear
View Solution play_arrow
-
question_answer54)
Beta rays emitted by a radioactive material are
A)
electromagnetic radiations done
clear
B)
the electrons orbiting around the nucleus done
clear
C)
charged particles emitted by the nucleus done
clear
D)
neutral particles done
clear
View Solution play_arrow
-
question_answer55)
Two radioactive materials \[{{X}_{1}}\] and \[{{X}_{2}}\] have decay constants \[10\lambda \] and \[\lambda \] respectively. If initially they have the same number of nuclei, then the ratio of the number of nuclei of \[{{X}_{1}}\] to that of \[{{X}_{2}}\] will be 1/e after a time
A)
\[\frac{1}{10\lambda }\] done
clear
B)
\[\frac{1}{11\lambda }\] done
clear
C)
\[\frac{11}{10\lambda }\] done
clear
D)
\[\frac{1}{9\lambda }\] done
clear
View Solution play_arrow
-
question_answer56)
The half-life of a radioactive isotope 'X' is 50 years. It decays to another element 'Y' which is stable. The two elements 'X' and 'Y were found to be in the ratio of 1:15 in a sample of a given rock. The age of the rock was estimated to be
A)
150 years done
clear
B)
200 years done
clear
C)
250 years done
clear
D)
100 years done
clear
View Solution play_arrow
-
question_answer57)
Let \[{{N}_{\beta }}\] be the number of p particles emitted by 1 gram of \[N{{a}^{24}}\] radioactive nuclei (half-life =15 hrs) in 7.5 hours, \[{{N}_{\beta }}\] is close to (Avogadro number \[=6.023\times {{10}^{23}}/g\text{ }mole\]):
A)
\[6.2\times {{10}^{21}}\] done
clear
B)
\[7.5\times {{10}^{21}}\] done
clear
C)
\[1.25\times {{10}^{22}}\] done
clear
D)
\[1.75\times {{10}^{22}}\] done
clear
View Solution play_arrow
-
question_answer58)
The activity of a radioactive sample is \[{{A}_{1}}\] at time \[{{t}_{1}}\] and \[{{A}_{2}}\] at time\[{{t}_{2}}\]. If \[\tau \] is average life of sample then the number of nuclei decayed in time (\[{{t}_{2}}-{{t}_{1}}\]) is
A)
\[{{A}_{1}}{{t}_{1}}-{{A}_{2}}{{t}_{2}}\] done
clear
B)
\[\frac{\left( {{A}_{2}}-{{A}_{1}} \right)}{2}\tau \] done
clear
C)
\[\left( {{A}_{1}}-{{A}_{2}} \right)\left( {{t}_{2}}-{{t}_{1}} \right)\] done
clear
D)
\[\left( {{A}_{1}}-{{A}_{2}} \right)\tau .\] done
clear
View Solution play_arrow
-
question_answer59)
One gram of a radioactive sample of half-life 10 min is sealed in a capsule at time t=0. Amount of sample decayed up to 5 min is
A)
0.293g done
clear
B)
0.5g done
clear
C)
0.25g done
clear
D)
0.707g done
clear
View Solution play_arrow
-
question_answer60)
Two radioactive substances A and B have decay constants \[5\lambda \] and \[\lambda \] respectively. At t=0 they have the same number of nuclei. The ratio of number of nuclei of A to those of B will be \[{{(1/e)}^{2}}\] after a time interval
A)
\[4\lambda \] done
clear
B)
\[2\lambda \] done
clear
C)
\[1/2\lambda \] done
clear
D)
\[1/4\lambda \] done
clear
View Solution play_arrow
-
question_answer61)
The half-life of radioactive Radon is 3.8 days. The time at the end of which \[\frac{1}{20}\]th of the radon sample will remain undecayed is \[\left( \text{given lo}{{\text{g}}_{e}}=0.4343 \right)\]
A)
3.8 days done
clear
B)
16.5 days done
clear
C)
33 days done
clear
D)
76 days. done
clear
View Solution play_arrow
-
question_answer62)
Average life of a radioactive sample is 4ms. Initially the total number of nuclei is \[{{N}_{0}}\]. A charged capacitor of capacity \[20\mu F\] is connected across a resistor R. The value of R such that ratio of number of nuclei remaining to charge on capacitor remains constant with time is
A)
100\[\Omega \] done
clear
B)
200 \[\Omega \] done
clear
C)
300 \[\Omega \] done
clear
D)
400 \[\Omega \] done
clear
View Solution play_arrow
-
question_answer63)
A radioactive nucleus can decay by two different processes. The half-life for the first process is \[{{t}_{1}}\] and that for the second process is \[{{t}_{2}}\]. If effective half-life is t, then
A)
\[t={{t}_{1}}+{{t}_{2}}\] done
clear
B)
\[\frac{1}{t}=\frac{1}{{{t}_{1}}}+\frac{1}{{{t}_{2}}}\] done
clear
C)
\[t=\frac{2{{t}_{1}}{{t}_{2}}}{{{t}_{1}}+{{t}_{2}}}\] done
clear
D)
\[t=\frac{{{t}_{1}}+{{t}_{2}}}{2}\] done
clear
View Solution play_arrow
-
question_answer64)
In a sample of rock, the ratio of \[^{206}Pb\] to \[^{238}U\] nuclei is found to be 0.5. The age in year of the rock is (given half-life of \[\,{{U}^{238}}\] is \[4.5\times {{10}^{9}}\] years)
A)
\[2.25\times {{10}^{9}}\] done
clear
B)
\[4.5\times {{10}^{9}}l\,\,n3\] done
clear
C)
\[4.5\times {{10}^{9}}\frac{ln\left( \frac{3}{2} \right)}{ln2}\] done
clear
D)
\[2.25\times {{10}^{9}}ln\left( \frac{3}{2} \right)\] done
clear
View Solution play_arrow
-
question_answer65)
In an \[\alpha \]-decay the kinetic energy of \[\alpha \]-particle is 48 MeV and Q-value of the reaction is 50 MeV. The mass number of the mother nucleus is (Assume that daughter nucleus is in ground state)
A)
96 done
clear
B)
100 done
clear
C)
104 done
clear
D)
110 done
clear
View Solution play_arrow
-
question_answer66)
If nuclei of a radioactive element is produced at constant rate \[\alpha \] and they decays with decay constant\[\lambda \]. At t=0, number of nuclei is zero than the number of nuclei at time t is
A)
\[\frac{\alpha }{\lambda }\left( 1-{{e}^{-\lambda t}} \right)\] done
clear
B)
\[\alpha -\frac{\alpha }{\lambda }{{e}^{-\lambda t}}\] done
clear
C)
\[\frac{\alpha }{\lambda }{{e}^{-\lambda t}}\] done
clear
D)
\[\alpha \left( 1-{{e}^{-\lambda t}} \right)\] done
clear
View Solution play_arrow
-
question_answer67)
Two radioactive substances X and Y emit \[\alpha \] and \[\beta \] particles respectively. Their disintegration constants are in the ratio 2 : 3. To have equal probabilities of getting emission of \[\alpha \] and \[\beta \] particles, the ratio of number of atoms X to that of Fat any time instant is
A)
2 : 3 done
clear
B)
3 : 2 done
clear
C)
e : 1 done
clear
D)
(e-1) : 1 done
clear
View Solution play_arrow
-
question_answer68)
A container is filled with a radioactive substance for which the half- life is 2 days. A week later, when the container is opened, it contains 5 g of the substance. Approximately how many grams of the substance were initially placed in the container?
A)
40 done
clear
B)
60 done
clear
C)
80 done
clear
D)
100 done
clear
View Solution play_arrow
-
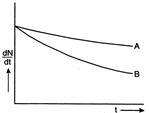
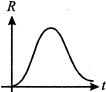
question_answer69)
Which sample, A or B shown in Fig. has shorter mean-life?
A)
A done
clear
B)
B done
clear
C)
both A and B have same mean-life done
clear
D)
Cannot be predicted done
clear
View Solution play_arrow
-
question_answer70)
The ratio of number of atoms of \[^{14}C\] to \[^{12}C\] in living matter is measured to be \[1.3\times {{10}^{-12}}\] at the present time. A 12 g sample of carbon produces 180 decays/min due to the small amount of \[^{14}C\] in it. The half-life of \[^{14}C\] is nearly [1 year \[=3.15\times {{10}^{7}}s\]]
A)
574 years done
clear
B)
5740 years done
clear
C)
2870 years done
clear
D)
287 years done
clear
View Solution play_arrow
-
question_answer71)
A radioactive substance with decay constant of \[0.5{{s}^{-1}}\] is being produced at a constant rate of 50 nuclei per second. If there are no nuclei present initially, the time (in second) after which 25 nuclei will be present is
A)
1 done
clear
B)
In 2 done
clear
C)
In (4/3) done
clear
D)
2 to (4/3) done
clear
View Solution play_arrow
-
question_answer72)
When the nucleus of a radium-226, which is at rest, decays, an \[\alpha \] particle and the nucleus of radon are created. The released energy during the decay is 4.87 MeV, which appears as the kinetic energy of the two resulted particles. Calculate the kinetic energy of a particle and radon nucleus.
A)
4.78 MeV and 0.09 MeV done
clear
B)
4.67 MeV and 0.2 MeV done
clear
C)
4.84 MeV and 0.03 MeV done
clear
D)
4.81 MeV and 0.06 MeV done
clear
View Solution play_arrow
-
question_answer73)
When a \[{{U}^{238}}\] nucleus originally at rest, decays by emitting an alpha particle having a speed the recoil speed of the residual nucleus is
A)
\[\frac{4u}{238}\] done
clear
B)
\[-\frac{5u}{234}\] done
clear
C)
\[\frac{4u}{234}\] done
clear
D)
\[-\frac{4u}{238}\] done
clear
View Solution play_arrow
-
question_answer74)
There are n number of radioactive nuclei in a sample that undergoes beta decay. If from the sample, n' number of P- particles are emitted every 2 s, then half- life of nuclei is
A)
\[n'/2\] done
clear
B)
\[0.693\times \left( 2n/n' \right)\] done
clear
C)
\[0.693\text{ }In\text{ }\left( 2n/n' \right)\] done
clear
D)
\[0.693\times n/n'\] done
clear
View Solution play_arrow
-
question_answer75)
A radioactive element decays to \[\frac{1}{4}\]th of its initial value in time t. The ratio of its half-life to mean life is
A)
\[\frac{\ln 2}{{{\lambda }^{2}}}\] done
clear
B)
\[\frac{\ln 2}{\lambda }\] done
clear
C)
\[2\ln 2\] done
clear
D)
\[\ln 2\] done
clear
View Solution play_arrow
-
question_answer76)
Starting with a sample \[^{66}Cu,\frac{7}{8}\] of pure decays into Zn in 15 minutes. The corresponding half-life is
A)
15 minutes done
clear
B)
10 minutes done
clear
C)
\[7\frac{1}{2}\]minutes done
clear
D)
5 minutes done
clear
View Solution play_arrow
-
question_answer77)
The luminous dials watches are usually made by mixing a zinc sulphide phosphor with an \[\alpha \]- particle emitter. The mass of radium (mass number 226, half-life 1620 years) that is needed to produce an average of 10 \[\alpha \]-particles per second for this purpose is
A)
\[2.77\text{ }mg\] done
clear
B)
\[2.77\text{ }g\] done
clear
C)
\[2.77\times {{10}^{-23}}g\] done
clear
D)
\[2.77\times {{10}^{-13}}kg\] done
clear
View Solution play_arrow
-
question_answer78)
A sample of radioactive substance has \[{{10}^{6}}\] nuclei. If half-life is 20 seconds, the number of nuclei left in the sample after 10 second is
A)
\[{{10}^{4}}\] done
clear
B)
\[2\times {{10}^{5}}\] done
clear
C)
\[7\times {{10}^{5}}\] done
clear
D)
\[11\times {{10}^{5}}\] done
clear
View Solution play_arrow
-
question_answer79)
The half-life of a radioactive element A is the same as the mean-life of another radioactive element B. Initially both substances have the same number of atoms, then:
A)
A and B decay at the same rate always. done
clear
B)
A and B decay at the same rate initially done
clear
C)
A will decay at a faster rate than B. done
clear
D)
B will decay at a faster rate than A. done
clear
View Solution play_arrow
-
question_answer80)
A piece of bone of an animal from a ruin is found to have \[^{14}C\] activity of 12 disintegrations per minute per gm of its carbon content. The \[^{14}C\] activity of a living animal is 16 disintegrations per minute per gm. How long ago nearly did the animal die? (Given half-life of \[^{14}C\] is \[{{t}_{1/2}}=5760\] years)
A)
1672 years done
clear
B)
2391 years done
clear
C)
3291 years done
clear
D)
4453 years done
clear
View Solution play_arrow
-
question_answer81)
An element A decays into an element C by a two-step process\[:\text{ }A\to B+He_{2}^{4}\] and \[B\to C+2e_{0}^{-1}\]Then
A)
A and C are isotopes done
clear
B)
A and C are isobars done
clear
C)
B and C are isotopes done
clear
D)
A and B are isobars done
clear
View Solution play_arrow
-
question_answer82)
Radium \[^{226}Ra,\] spontaneously decays to radon with the emission of an \[\alpha -\]particle and a \[\gamma \] ray. If the speed of the \[\alpha \] particle upon emission from an initially stationary radium nucleus is \[1.5\times {{10}^{7}}m/s,\] what is the recoil speed of the resultant radon nucleus? Assume the momentum of \[\gamma \]ray is negligible compared to that of \[\alpha \]particle.
A)
\[1.6\times {{10}^{4}}m/s\] done
clear
B)
\[2.7\times {{10}^{5}}m/s\] done
clear
C)
\[1.3\times {{10}^{6}}m/s\] done
clear
D)
\[2.6\times {{10}^{3}}m/s\] done
clear
View Solution play_arrow
-
question_answer83)
In a certain hypothetical radioactive decay process, species A decays into species B and species B decays into species C according to the reactions: \[A\xrightarrow{{}}2B+particles+energy\] \[B\xrightarrow{{}}3C+particles+energy\] The decay constant for species is \[{{\lambda }_{1}}=1{{\sec }^{-1}}\] and that for the species B is \[{{\lambda }_{2}}=100{{\sec }^{-1}}.\] Initially \[{{10}^{4}}\]moles of species of A were present while there was none of B and C. It was found that species B reaches its maximum number of moles of B.
A)
1 done
clear
B)
2 done
clear
C)
5 done
clear
D)
4 done
clear
View Solution play_arrow
-
question_answer84)
A radioactive isotope has a half-life of 200 years. How long will it take the activity to reduce to 1% of its original value?
A)
330 years done
clear
B)
460 years done
clear
C)
660 years done
clear
D)
920 years done
clear
View Solution play_arrow
-
question_answer85)
A radioactive nucleus (initial mass number A and atomic number Z emits 3 \[\alpha -\]particles and 2 positrons. The ratio of number of neutrons to that of protons in the final nucleus will be
A)
\[\frac{A-Z-8}{Z-4}\] done
clear
B)
\[\frac{A-Z-4}{Z-8}\] done
clear
C)
\[\frac{A-Z-12}{Z-4}\] done
clear
D)
\[\frac{A-Z-4}{Z-2}\] done
clear
View Solution play_arrow
-
question_answer86)
The half life of a radioactive substance is 20 minutes. The approximate time interval \[({{t}_{2}}-{{t}_{1}})\]between the time \[{{t}_{2}}\] when \[\frac{2}{3}\] of it had decayed and time \[{{t}_{1}}\] when \[\frac{1}{3}\] of it had decayed is:
A)
14 min done
clear
B)
20 min done
clear
C)
28 min done
clear
D)
7 min done
clear
View Solution play_arrow
-
question_answer87)
After absorbing a slowly moving neutron of mass \[{{m}_{N}}\] (momentum\[\approx 0\]) a nucleus of mass M breaks into two nuclei masses \[{{m}_{1}}\] and \[5{{m}_{1}}\]\[(6{{m}_{1}}=M\,+{{m}_{N}})\] respectively. If the de Broglie wavelength of the nucleus with mass \[{{m}_{1}}\] is \[\lambda ,\] the de Broglie wavelength of the nucleus will be
A)
\[5\lambda \] done
clear
B)
\[\lambda /5\] done
clear
C)
\[\lambda \] done
clear
D)
\[25\lambda \] done
clear
View Solution play_arrow
-
question_answer88)
Assume that a neutron breaks into a proton and an electron. The energy released during this process is: (mass of neutron \[=1.6725\times {{10}^{-27}}kg,\] mass of proton \[=1.6725\times {{10}^{-27}}kg,\] mass of electron \[=9\times {{10}^{-31}}kg\]).
A)
\[0.51\,\,MeV\] done
clear
B)
\[7.10\,\,MeV\] done
clear
C)
\[6.30\,\,MeV\] done
clear
D)
\[5.4\,\,MeV\] done
clear
View Solution play_arrow
-
question_answer89)
Half-lives of two radioactive elements A and B are 20 minutes and 40 minutes, respectively. Initially, the samples have equal number of nuclei. After 80 minutes, the ratio of decayed number of A and B nuclei will be:
A)
\[1:4\] done
clear
B)
\[5:4\] done
clear
C)
\[1:16\] done
clear
D)
\[4:1\] done
clear
View Solution play_arrow
-
question_answer90)
A radioactive nucleus A with a half-life T, decays into a nucleus B. At \[t=0,\]there is no nucleus B. At some time t, the ratio of the number of B to that of A is 0.3. Then, t is given by
A)
\[t=T\log (1.3)\] done
clear
B)
\[t=\frac{T}{\log (1.3)}\] done
clear
C)
\[t=T\frac{\log 2}{\log 1.3}\] done
clear
D)
\[t=\frac{\log 1.3}{\log 2}T\] done
clear
View Solution play_arrow
-
question_answer91)
A \[^{7}Li\] target is bombarded with a proton beam current of \[{{10}^{-4}}\] A for 1 hour to produce \[^{7}Be\] of activity \[1.8\times {{10}^{8}}\] disintegrations per second. Assuming that one \[^{7}Be\] radioactive nucleus is produced by bombarding 1000 protons, determine its half-life.
A)
\[8.6\times {{10}^{6}}s\] done
clear
B)
\[4.2\times {{10}^{5}}s\] done
clear
C)
\[3.1\times {{10}^{5}}s\] done
clear
D)
\[1.1\times {{10}^{6}}s\] done
clear
View Solution play_arrow
-
question_answer92)
The disintegration rate of a certain radioactive sample at any instant is 4750 disintegrations per minute. Five minutes later the rate becomes 2700 disintegrations per minute. Calculate half-life of the sample.
A)
\[9.1\,\min \] done
clear
B)
\[6.1\,\min \] done
clear
C)
\[2.3\,\min \] done
clear
D)
\[1.1\,\min \] done
clear
View Solution play_arrow
-
question_answer93)
A nucleus at rest undergoes a decay emitting an \[\alpha -\]particle of de-Broglie wavelength \[\lambda =5.76\times {{10}^{-15}}m.\] If the mass of the daughter nucleus is \[223.610\] amu and that of the \[\alpha -\]particle is \[4.002\,amu,\] determine the mass of the parent nucleus inamu. \[(1\,amu=931.470\,\,MeV/{{c}^{2}}].\]
A)
\[227.62\,\,amu\] done
clear
B)
\[112.11\,\,amu\] done
clear
C)
\[90.3\,\,amu\] done
clear
D)
\[23.8\,\,amu\] done
clear
View Solution play_arrow
-
question_answer94)
In an ore containing uranium, the ratio of \[{{U}^{238}}\] to \[P{{b}^{206}}\]nuclei is 3. Calculate the age of the ore, assuming that all the lead present in the ore in the final stable product of \[{{U}^{238}}\]. Take the half-life of \[{{U}^{238}}\]to be \[4.5\times {{10}^{9}}\]ear.
A)
\[1.8\times {{10}^{9}}\] year done
clear
B)
\[2.3\times {{10}^{10}}\]year done
clear
C)
\[5.1\times {{10}^{7}}\] year done
clear
D)
\[6.2\times {{10}^{6}}\]year done
clear
View Solution play_arrow
-
question_answer95)
The proton -proton mechanism that accounts for energy production in the sun releases \[26.7\text{ }MeV\]energy for each event. In this process, protons fuse to form an alpha particle \[{{(}^{4}}He)\]. At what rate \[\frac{dm}{dt}\] is hydrogen being consumed in the core of the sun by the p-p cycle? Power of sun is\[3.90\times {{10}^{26}}W\].
A)
\[1.6\times {{10}^{10}}kg/s\] done
clear
B)
\[2.3\times {{10}^{9}}kg/s\] done
clear
C)
\[6.2\times {{10}^{11}}\,kg/s\] done
clear
D)
\[5.5\times {{10}^{10}}\,kg/s\] done
clear
View Solution play_arrow
-
question_answer96)
Consider the fusion in helium plasma. Find the temperature at which the average thermal energy \[1.5kT\] equals the coulomb potential energy at 2 fin.
A)
\[1.2\times {{10}^{5}}K\] done
clear
B)
\[2.23\times {{10}^{10}}K\] done
clear
C)
\[5.4\times {{10}^{3}}K\] done
clear
D)
\[1.1\times {{10}^{5}}K\] done
clear
View Solution play_arrow
-
question_answer97)
The element curium \[_{96}C{{m}^{248}}\] has a mean life of \[{{10}^{13}}s.\]. Its primary decay modes are spontaneous fission and \[\alpha \]-decay, the former with a probability of \[8%\] and the later with a probability of \[92%\]. Each fission releases \[200\text{ }MeV\] of energy. The masses involved in a decay are as follows: \[_{96}C{{m}^{248}}=248.072220u,\]\[_{94}P{{u}^{244}}=244.064100\,u\] and \[_{2}H{{e}^{4}}=4.002603u\] Calculate the power output from a sample of \[{{10}^{20}}Cm\] atoms. \[(1\,u=931\,\,MeV/{{c}^{2}}).\]
A)
\[1.6\times {{10}^{-5}}W\] done
clear
B)
\[2.6\times {{10}^{-3}}W\] done
clear
C)
\[3.3\times {{10}^{-5}}W\] done
clear
D)
\[5.1\times {{10}^{-3}}W\] done
clear
View Solution play_arrow
-
question_answer98)
A radioactive element decays by p emission. A detector records n beta particles in 2 second and in next 2 seconds it records \[0.75\text{ }n\] beta particles. Find mean life corrected to nearest whole number. Given \[\ell n2=0.6931\] and \[\ell n3=1.0986.\].
A)
\[6.9\text{ }sec\] done
clear
B)
\[9.9\text{ }sec\] done
clear
C)
\[10.1\text{ }sec\] done
clear
D)
\[12.2\text{ }sec\] done
clear
View Solution play_arrow
-
question_answer99)
The count rate from a radioactive sample falls from \[4.0\times {{10}^{6}}\] per second to \[1\times {{10}^{6}}\]per second in 20 hour. What will be the count rate, 100 hour after the beginning?
A)
\[3.91\times {{10}^{3}}\text{ }se{{c}^{-1}}\] done
clear
B)
\[5.81\times {{10}^{4}}\text{ }se{{c}^{-1}}\] done
clear
C)
\[6.22\times {{10}^{5}}\text{ }se{{c}^{-1}}\] done
clear
D)
None of these done
clear
View Solution play_arrow
-
question_answer100)
The ratio of half-life times of two elements A and B is \[\frac{{{T}_{A}}}{{{T}_{B}}}\]. The ratio of respective decay constant \[\frac{{{\lambda }_{A}}}{{{\lambda }_{B}}},\] is
A)
\[{{T}_{B}}/{{T}_{A}}\] done
clear
B)
\[{{T}_{A}}/{{T}_{B}}\] done
clear
C)
\[\frac{{{T}_{A}}+{{T}_{B}}}{{{T}_{A}}}\] done
clear
D)
\[\frac{{{T}_{A}}-{{T}_{B}}}{{{T}_{A}}}\] done
clear
View Solution play_arrow
 done
clear
done
clear
 done
clear
done
clear
 done
clear
done
clear
 done
clear
done
clear

