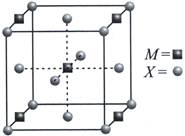
| A compound \[{{M}_{p}}{{X}_{q}}\] has cubic close packing (ccp) arrangement of X. Its unit cell structure shown below. The empirical formula of the compound is |
 |
A) MX
B) \[M{{X}_{2}}\]
C) \[{{M}_{2}}X\]
D) \[{{M}_{5}}{{X}_{14}}\]
Correct Answer: B
Solution :
8 X atoms present at the corners. Atoms contribute to 1 unit cell \[=\frac{1}{8}\times 8=1\] 6X atoms present at the face centres. Atoms contribute to 1 unit cell=\[6\times \frac{1}{2}=3\] Total X atoms = 3 + 1 = 4 4M atoms present at edge centres. Atoms present in 1 unit cell \[=4\times \frac{1}{4}=1\] 1M atom present at body centre and it contributes completely to 1 unit cell. Thus, total M atoms in one unit cell =1+1=2 Ratio is M : X : : 2 : 4 : : 1 : 2 Thus, empirical formula is \[M{{X}_{2}}\]You need to login to perform this action.
You will be redirected in
3 sec
