| (i) V is a reactive metal. |
| (ii) X is a reactive non-metal. |
| (iii) U is a noble gas. |
A) (i) and (ii) only
B) (ii) and (iii) only
C) (i) and (iii) only
D) All of these
Correct Answer: D
Solution :
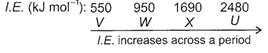 As we move across a period from left to right, ionization energy increases due to decrease in atomic size. V is a reactive alkali metal which has low value of first ionization energy and loses one electron easily to form monovalent cation. X is a reactive non-metal which has high value of first ionization energy thus, it prefers to gain electron rather than losing. U having very high value of ionization energy is a noble gas which has stable, completely filled electronic configuration and is chemically inert.
As we move across a period from left to right, ionization energy increases due to decrease in atomic size. V is a reactive alkali metal which has low value of first ionization energy and loses one electron easily to form monovalent cation. X is a reactive non-metal which has high value of first ionization energy thus, it prefers to gain electron rather than losing. U having very high value of ionization energy is a noble gas which has stable, completely filled electronic configuration and is chemically inert.
You need to login to perform this action.
You will be redirected in
3 sec
