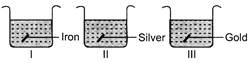
A)
I II III Green Blue Green
B)
I II III Blue Green Green
C)
I II III Green Blue Blue
D)
I II III Blue Blue Blue
Correct Answer: C
Solution :
As iron is more reactive than copper, it displaces copper from copper sulphate solution and green solution of iron sulphate is formed. \[Fe+\underset{\begin{smallmatrix} Blue\,coloured\, \\ solution \end{smallmatrix}}{\mathop{CuS{{O}_{4}}}}\,\to \underset{\begin{smallmatrix} Green\,\,coloured \\ solution \end{smallmatrix}}{\mathop{FeS{{O}_{4}}}}\,+Cu\] Silver and gold are less reactive than copper. So, they do not displace copper from copper sulphate solution. Hence, solution remains blue in colour.You need to login to perform this action.
You will be redirected in
3 sec
