A) In electrophilic substitution reaction amino group is meta directive
B) Inspite of substituents nitro group always goes to m- position
C) In strong acidic medium, nitration of aniline is a nucleophic substitution reaction
D) In strong acidic medium aniline present as anilinium ion
E) Strong acid, gives nitrate anion, which attacks at m-position
Correct Answer: D
Solution :
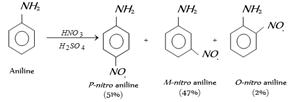 The reason for this is that, in acidic condition protonation of \[-N{{H}_{2}}\] group gives anilinium ion \[(+N{{H}_{3}})\], which is of deactivating nature and of m-directive nature.
The reason for this is that, in acidic condition protonation of \[-N{{H}_{2}}\] group gives anilinium ion \[(+N{{H}_{3}})\], which is of deactivating nature and of m-directive nature.
You need to login to perform this action.
You will be redirected in
3 sec
