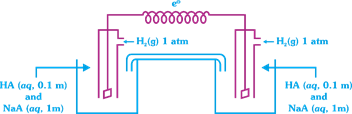
A) 0.81 V
B) 0.071 V
C) 0.0591 V
D) 1.182 V
Correct Answer: C
Solution :
| [c] \[{{E}_{cell}}=\frac{0.0591}{1}\log \frac{{{[{{H}^{+}}]}_{RHS}}}{{{[{{H}^{+}}]}_{LHS}}}\] |
| \[{{E}_{cell}}=0.0591[p{{H}_{LHS}}-p{{H}_{RHS}}]\] |
| \[p{{H}_{LHS}}p{{K}_{a}}+\log \frac{[{{A}^{-}}]}{[HA]}\] |
| \[=8+\log \frac{1}{0.1}=9\] \[p{{H}_{RHS}}=8\] |
| \[{{E}_{cell}}=0.0591(9-8)=0.0591V\] |
You need to login to perform this action.
You will be redirected in
3 sec
