| DIRECTION: Read the passage given below and answer the questions that follows: |
| In the figure n mole of a monoatomic ideal gas undergo the process ABC as shown in the P-V diagram. The process AB is isothermal and BC is isochoric. The temperature of the gas at A is \[{{T}_{0}}\]. Total heat given to the gas during the process ABC is measured to be Q. |
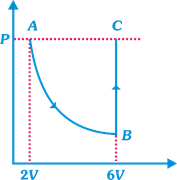 |
| Temperature of the gas at C is equal to |
A) \[{{T}_{0}}\]
B) \[3{{T}_{0}}\]
C) \[6{{T}_{0}}\]
D) \[2{{T}_{0}}\]
Correct Answer: B
Solution :
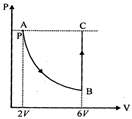
| [b] AB is an isothermal process then |
| \[P\times 2V={{P}_{B}}\times 6V\Rightarrow {{P}_{B}}=\frac{P}{3}\] |
 |
| Now, BC is an isochoric process then |
| \[\frac{{{P}_{B}}}{{{T}_{B}}}=\frac{{{P}_{C}}}{{{T}_{C}}};\frac{P}{3{{T}_{0}}}=\frac{P}{{{T}_{C}}};{{T}_{C}}=3{{T}_{0}}\] |
You need to login to perform this action.
You will be redirected in
3 sec
