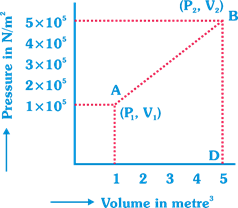
A) \[7.5\times {{10}^{5}}\] joule
B) \[7.5\times {{10}^{5}}\] ergs
C) \[12\times {{10}^{5}}\] joule
D) \[6\times {{10}^{5}}\] joule
Correct Answer: C
Solution :
[c] Work done = Area of graph ABD; From above we get, Area \[=1/2\times 4(1\times {{10}^{5}}+5\times {{10}^{5}})\]\[=2\times 6\times {{10}^{5}}=12\times {{10}^{5}}\text{J}\].You need to login to perform this action.
You will be redirected in
3 sec
